Adeno-Associated Viruses as Gene Therapy Vectors: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
[[Image:AAV TEM.jpg|thumb|185px|right|Electron micrograph of the Adeno-Associated Virus.]] | |||
==Adeno-Associated Viruses== | ==Adeno-Associated Viruses== | ||
<br>Adeno-associated viruses (AAV) small viruses that affect humans and other members of the primate species. They belong to the parovirus family and require a helper virus, such as adenovirus, herpes simplex virus, vaccina virus, or CMV, to replicate. For this particular reason they are also part of the dependovirus genus. If no helper virus is present, they are able to incorporate themselves into the host cell’s genome and latently replicate as the host cell replicates. When a helper virus infects the cells that the AAV has latently incorporated itself into, it is able to engage in lytic replication and release itself from the cell. | <br>Adeno-associated viruses (AAV) small viruses that affect humans and other members of the primate species. They belong to the parovirus family and require a helper virus, such as adenovirus, herpes simplex virus, vaccina virus, or CMV, to replicate. For this particular reason they are also part of the dependovirus genus. If no helper virus is present, they are able to incorporate themselves into the host cell’s genome and latently replicate as the host cell replicates. When a helper virus infects the cells that the AAV has latently incorporated itself into, it is able to engage in lytic replication and release itself from the cell. <br> | ||
<br> | |||
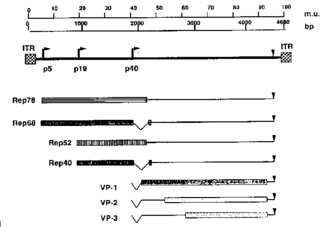
[[Image:AAV genome.gif|thumb|325px|right|Genome of AAV.]] | [[Image:AAV genome.gif|thumb|325px|right|Genome of AAV.]] | ||
<br>The AAV genome is made up of ssDNA, is very small (4.7kbp), and is flanked by two 145 nucleotide-long inverted terminal repeats. It codes for two different types of proteins, the first being Rep, which is responsible for replication and rescue of the virus. The second protein, Cap, is a structural protein that is the chief constituent of the icosahedral capsid that houses the viral genome. Furthermore, the AAV genome contains three promoters, p5, p19, and p40, and two open reading frames. The mRNA transcripts produced in conjunction with each of the promoters is able to code for seven different Rep and Cap proteins due to alternate splicing. AAV’s genome doesn’t code for a DNA polymerase and is consequently dependent on that provided by the host cell. AAV are very simple viruses, but as we will see, they have an extremely wide variety of medicinal applications.<br> | |||
| Line 12: | Line 13: | ||
<br>The only portions of the AAV that are absolutely necessary in cis to the vector are the inverted terminal repeats and the therapeutic gene. The packaging and structural genes, rep and cap, are able to be provided in trans. This means that the genome of the AAV can essentially be wiped out to make room for recombinant genes.<br> | <br>The only portions of the AAV that are absolutely necessary in cis to the vector are the inverted terminal repeats and the therapeutic gene. The packaging and structural genes, rep and cap, are able to be provided in trans. This means that the genome of the AAV can essentially be wiped out to make room for recombinant genes.<br> | ||
==Using | ==Using AAV Vectors to Treat Diseases== | ||
<br>AAV vectors have been shown to be capable or targeting a wide variety of diseases and malignancies. Common diseases targeted include various types of cancer, neurodegenerative diseases, arthritis, muscular dystrophy, cystic fibrosis, hemophilia B, and many more. <br> | <br>AAV vectors have been shown to be capable or targeting a wide variety of diseases and malignancies. Common diseases targeted include various types of cancer, neurodegenerative diseases, arthritis, muscular dystrophy, cystic fibrosis, hemophilia B, and many more. <br> | ||
Revision as of 01:26, 16 April 2009
Adeno-Associated Viruses
Adeno-associated viruses (AAV) small viruses that affect humans and other members of the primate species. They belong to the parovirus family and require a helper virus, such as adenovirus, herpes simplex virus, vaccina virus, or CMV, to replicate. For this particular reason they are also part of the dependovirus genus. If no helper virus is present, they are able to incorporate themselves into the host cell’s genome and latently replicate as the host cell replicates. When a helper virus infects the cells that the AAV has latently incorporated itself into, it is able to engage in lytic replication and release itself from the cell.
The AAV genome is made up of ssDNA, is very small (4.7kbp), and is flanked by two 145 nucleotide-long inverted terminal repeats. It codes for two different types of proteins, the first being Rep, which is responsible for replication and rescue of the virus. The second protein, Cap, is a structural protein that is the chief constituent of the icosahedral capsid that houses the viral genome. Furthermore, the AAV genome contains three promoters, p5, p19, and p40, and two open reading frames. The mRNA transcripts produced in conjunction with each of the promoters is able to code for seven different Rep and Cap proteins due to alternate splicing. AAV’s genome doesn’t code for a DNA polymerase and is consequently dependent on that provided by the host cell. AAV are very simple viruses, but as we will see, they have an extremely wide variety of medicinal applications.
AAV Vector Production (2 Transfection Method)
The first step in producing AAV vectors is finding helper viruses that will aid in replicating the AAV. Many times this helper virus is an adenovirus, which needs a minimum of its E2A, E4, and VA1/II gene regions in order to be effective at replicating the AAV. Then, two plasmids are created, one containing the AAV rep and cap genes plus the adenoviral helper genes prescribed above. The other plasmi containins an expression cassette composed of a CMV promoter, multiple cloning site, and SV40 polyA. This expression cassette on the second plasmid is also flanked by AAV inverted terminal repeats. This concoction is then transfected into a large quantity of 150mm dishes that contain the 293 line of human embryonic kidney cells, which encode the E1 region of the Ad5 genome. After the transfection, the cells are harvested and purified using ultracentrifugation. Another advantage of the AAV system is that large number of AAV can be harvested using these techniques (>10^8vp/ml).
The only portions of the AAV that are absolutely necessary in cis to the vector are the inverted terminal repeats and the therapeutic gene. The packaging and structural genes, rep and cap, are able to be provided in trans. This means that the genome of the AAV can essentially be wiped out to make room for recombinant genes.
Using AAV Vectors to Treat Diseases
AAV vectors have been shown to be capable or targeting a wide variety of diseases and malignancies. Common diseases targeted include various types of cancer, neurodegenerative diseases, arthritis, muscular dystrophy, cystic fibrosis, hemophilia B, and many more.
Functionally, AAV are well suited as gene vectors. One important aspect of their function is that they are able to transduce a wide variety of cells regardless of their stage in the cell cycle. This is extremely important because it allows them to target cells that are differentiated, and also cells that are rapidly dividing, such as cancer cells. Furthermore, AAV are desirable as gene vectors because when they infect host cells, they elicit little immune response. If any response is elicited, it is little more than the generation of neutralizing antibodies. No cytotoxic effect is caused, and the host system is relatively unaffected other than the intended, therapeutic, infection. All of these aspects of AAV are important, but probably the greatest advantage of the AAV vector system is that it allows for permanent genetic implantations to occur. Unlike the adenovirus, AAV is able to permanently implant its genes into the genome of the host, which creates a relatively-long term effect on the host as opposed to a powerful, transient, effect like that typically produced by adenoviral vectors. AAV are also extremely reliable and predictable when inserting their genes into the host cell’s genome. They always implant their genes into a particular spot on the 19th chromosome of humans, while retroviruses are extremely unpredictable and prone to mutating DNA during implantation. If mutations or misplaced insertions occur at this stage of the therapy, malignancies and adverse effects may be observed, compromising the efficacy of the treatment. There are also 11 different serotypes of AAV that are all different in their structure. Certain serotypes, such as AVV2, are specific to particular receptors in the host. This aids researchers to mix and match the genomes of these serotypes to find a vector that is specific to a particular receptor in the host. All of these are advantages make using AAV very desirable for a wide variety of gene therapy treatments.
While there are a great amount of advantages to using the AAV vector system, there are also a few drawbacks. The main drawback of using AAV as vectors is that the small size of their genome (4kbp) significantly limits the amount of genetic material that it can carry. Oftentimes, almost the entire genome must be replaced in order for the required genes to be placed onto the AAV. By comparing the size of the AAV genome to other types of vectors it becomes evident as to why it is so disadvantageous. Adenoviruses, for example, can carry roughly twice as much material and retroviruses usually hold between 8-10kbp. While the AAV is unable to carry large genes, steps are being taken to discover ways to expand the carrying capacity of AAV vectors by annealing the two inverted terminal repeats (ITR).
Section 3
Include some current research in each topic, with at least one figure showing data.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Chad Kurylo for BIOL 238 Microbiology, 2009, Kenyon College.