Bacillus Anthracis: Anthrax Lethal Toxin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
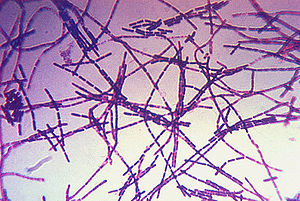
<p>Anthrax is a disease that typically affects herbivores but can infect any mammal, including humans. It is caused by the bacterium Bacillus anthracis (B. anthracis). This disease has become a hot topic in today’s society, in which terrorism is becoming more prevalent, as it can be used in biological warfare. Recently, there has also been an increase in the number of cases of injection anthrax, a form of the disease that affects heroin users, in Europe (Grunow). However, this disease is not new. In fact, there is evidence of it throughout history. The fifth and sixth plagues in Egypt (Exodus, Chapter 9) have been attributed to this pathogen (Baillie). | <p>[[Image:Bacillus_anthracis_gram.jpg|thumb|300px|right|A photomicrograph of Bacillus anthracis bacteria using Gram-stain technique. Photo obtained from the CDC's Public Health Image Library.]]</p> | ||
<p>Anthrax is a disease that typically affects herbivores but can infect any mammal, including humans. It is caused by the bacterium Bacillus anthracis (B. anthracis). This disease has become a hot topic in today’s society, in which terrorism is becoming more prevalent, as it can be used in biological warfare. Recently, there has also been an increase in the number of cases of injection anthrax, a form of the disease that affects heroin users, in Europe (Grunow). However, this disease is not new. In fact, there is evidence of it throughout history. The fifth and sixth plagues in Egypt (Exodus, Chapter 9) have been attributed to this pathogen (Baillie). </p> | |||
<p>The species derives its name from the Greek word anthrakis, meaning coal, because infection by the organism causes the formation of black, cutaneous eschars (Spencer). There are are three types of B. anthracis infection that are typically described: cutaneous anthrax (the cause of the black sores), inhalation anthrax (the most deadly form), and gastrointestinal anthrax (Mock, Mignot).</p> | <p>The species derives its name from the Greek word anthrakis, meaning coal, because infection by the organism causes the formation of black, cutaneous eschars (Spencer). There are are three types of B. anthracis infection that are typically described: cutaneous anthrax (the cause of the black sores), inhalation anthrax (the most deadly form), and gastrointestinal anthrax (Mock, Mignot).</p> | ||
==Organism== | ==Organism== | ||
| Line 41: | Line 36: | ||
==Pathogenesis== | ==Pathogenesis== | ||
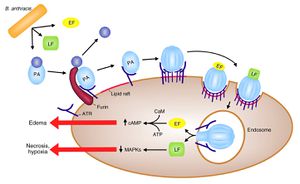
<p>[[Image:BAnthracisPathogenesis.jpg|thumb|300px|right|Figure 2. The process of <i>B. anthracis</i> pathogenesis begins with the binding of PA to a host cell. The PA molecule is then cleaved and oligomerizes, at which time it can bind LF and EF forming LeTx and EdTx. These complexes are then internalized into the cell, where LeTx causes reduced intracellular MAPK concentration, resulting in necrosis and hypoxia, and EdTx causes increased intracellular cAMP concentration, resulting in edema around the infection site.]]</p> | |||
<p>As noted above, infection by B. anthracis begins with the germination of a spore in the host organism. When germination occurs, the vegetative bacteria enter the blood stream and begin rapid extracellular multiplication. At this time, synthesis and extracellular secretion of capsule proteins and exotoxins also begins.</p> | <p>As noted above, infection by B. anthracis begins with the germination of a spore in the host organism. When germination occurs, the vegetative bacteria enter the blood stream and begin rapid extracellular multiplication. At this time, synthesis and extracellular secretion of capsule proteins and exotoxins also begins.</p> | ||
<p>The exotoxins of B. anthracis act in binary combinations (LF + PA and EF + PA) to form lethal toxin (LeTx) and edema toxin (EdTx). Protective antigen molecules act by binding to a receptor on a target cell, inserting into the cell membrane, and translocating the bound toxin factor into the cytosol of the target cell (Langer). This is accomplished by the binding of PA to anthrax toxin receptor (ATR), a membrane protein located in many cell types. PA is then cleaved by a furin-family protease to reveal the LF and EF binding sites. PA then oligomerizes into a hepatomer and binds LF or EF, a competitive process between the two toxin factors. The newly formed LeTx or EdTx complexes are activated by acidic conditions in an endosome when they enter the cell via endocytosis, and they are then transferred into the cytosol.</p> | <p>The exotoxins of B. anthracis act in binary combinations (LF + PA and EF + PA) to form lethal toxin (LeTx) and edema toxin (EdTx). Protective antigen molecules act by binding to a receptor on a target cell, inserting into the cell membrane, and translocating the bound toxin factor into the cytosol of the target cell (Langer). This is accomplished by the binding of PA to anthrax toxin receptor (ATR), a membrane protein located in many cell types. PA is then cleaved by a furin-family protease to reveal the LF and EF binding sites. PA then oligomerizes into a hepatomer and binds LF or EF, a competitive process between the two toxin factors. The newly formed LeTx or EdTx complexes are activated by acidic conditions in an endosome when they enter the cell via endocytosis, and they are then transferred into the cytosol.</p> | ||
<p>Inside the cell, EF is an adenylate cyclase, which converts intracellular ATP into cAMP, a process that is dependent on the protein calmodulin, which is produced by the host cell and acts as a ligand for EF. This process causes a dramatic increase in intracellular cAMP, disrupting cell signalling as well as membrane permeability regulation, which leads to edema at and around the infection site (Chung). In the case of inhalation anthrax, this edema presents as pleural effusion. Lethal factor is a zinc metalloprotease that cleaves the N-terminus of mitogen-activated protein kinases (MAPKKs), which are involved in cell signaling pathways (Mock, Mignot). This process is illustrated in Figure 2. The exact physiological mechanisms by which LeTx and EdTx kill the organism are not yet known.</p> | <p>Inside the cell, EF is an adenylate cyclase, which converts intracellular ATP into cAMP, a process that is dependent on the protein calmodulin, which is produced by the host cell and acts as a ligand for EF. This process causes a dramatic increase in intracellular cAMP, disrupting cell signalling as well as membrane permeability regulation, which leads to edema at and around the infection site (Chung). In the case of inhalation anthrax, this edema presents as pleural effusion. Lethal factor is a zinc metalloprotease that cleaves the N-terminus of mitogen-activated protein kinases (MAPKKs), which are involved in cell signaling pathways (Mock, Mignot). This process is illustrated in Figure 2. The exact physiological mechanisms by which LeTx and EdTx kill the organism are not yet known.</p> | ||
| Line 70: | Line 66: | ||
==Anthrax Toxin Neutralization with Antibody== | ==Anthrax Toxin Neutralization with Antibody== | ||
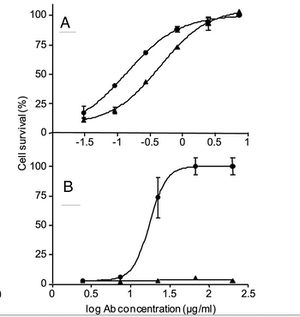
<p> | <p>[[Image:cAb29Fig.jpg|thumb|300px|right|Figure 1. A and B show the neutralization assay performed using cultured J774A macrophages that were incubated for 5 h with fixed amounts of LF and PA83 (A) or purified prepore (B) in the presence of cAb29 (circles) or Ab33 (triangles) in the indicated concentrations. Cell survival was determined by XTT and plotted as the percentage of untreated control cells. Points are mean ± S.D. of triplicate determinants. Ab concentration, antibody concentration (Mechaly).]]</p> | ||
<p>During infection by B. anthracis, protective antigen is integral in the intoxication of the host cells by anthrax toxins. This protein, therefore, is a logical target for scientists seeking to neutralize the anthrax toxins. Mechaly, et al. have recently found that the antibody cAb29, does just that: it targets PA molecules, interfering with the function of the molecule and thereby neutralizing the anthrax toxin (Mechaly).</p> | |||
<p>The Mechaly group, in attempting to determine the mechanism by which cAb29 functions to neutralize the toxins, found that cAb29 does not have any effect on the initial steps of the intoxication process: PA molecules are still able to bind to the target cell via receptors on the target cell membrane. These molecules are still cleaved by the furin-family protease and they still oligomerize into hepatomers which bind EF and LF molecules. Instead, they discovered that the antibody binds to the prepore, which is the complex of PA and either LF or EF before it is internalized by the cell, preventing the acid catalyzed transition to the transmembranal pore (Mechaly). </p> | <p>The Mechaly group, in attempting to determine the mechanism by which cAb29 functions to neutralize the toxins, found that cAb29 does not have any effect on the initial steps of the intoxication process: PA molecules are still able to bind to the target cell via receptors on the target cell membrane. These molecules are still cleaved by the furin-family protease and they still oligomerize into hepatomers which bind EF and LF molecules. Instead, they discovered that the antibody binds to the prepore, which is the complex of PA and either LF or EF before it is internalized by the cell, preventing the acid catalyzed transition to the transmembranal pore (Mechaly). </p> | ||
| Line 76: | Line 74: | ||
<p>Their tests showed 100% survival of mice injected intravenously with cAb29 12 hours after initial exposure to B. anthracis (Figure 1). This is a very important discovery as it could possibly lead to a cure for the disease even if it is not caught in the very early stages of infection (Mechaly).</p> | <p>Their tests showed 100% survival of mice injected intravenously with cAb29 12 hours after initial exposure to B. anthracis (Figure 1). This is a very important discovery as it could possibly lead to a cure for the disease even if it is not caught in the very early stages of infection (Mechaly).</p> | ||
[[Image: | ==Target Cells in Humans in Inhalation Anthrax== | ||
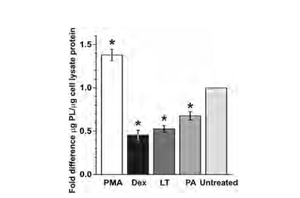
<p>[[Image:surfactantFig.png|thumb|300px|right|LeTx decreases surfactant production in AEC. Early-culture AEC were treated with LeTx (2 micro grams/ml) for 72 h and harvested. Phorbol 12-myristate (PMA; 50 ng/ml) and dexamethasone (Dex; 1 microM) were used as positive and negative controls, respectively. Surfactant levels in supernatants were measured by a phospholipid assay and normalized to protein content per sample. Data are means SEM of the results from 3 experiments on 3 separate individuals, with 2 to 4 trials for each treatment. *, P value of <0.05 versus untreated cells (Langer).]]</p> | |||
<p>Until recently, scientists believed that alveolar macrophages were the target cells for inhaled B. anthracis spores in humans because the disease had been previously shown to inhibit mouse alveolar macrophages. However, more recent studies have determined that human alveolar macrophages do not express the anthrax toxin receptor protein and are therefore are unaffected by anthrax toxins because PA molecules are unable to bind to the cells. In their 2012 paper, Langer et al. explain how they determined what cells are actually the target of anthrax toxin is in humans. The results that they obtained show that human alveolar epithelial cells (AECs) express the anthrax toxin receptor, and they are, therefore, the target cell in the human respiratory system (Langer).</p> | <p>Until recently, scientists believed that alveolar macrophages were the target cells for inhaled B. anthracis spores in humans because the disease had been previously shown to inhibit mouse alveolar macrophages. However, more recent studies have determined that human alveolar macrophages do not express the anthrax toxin receptor protein and are therefore are unaffected by anthrax toxins because PA molecules are unable to bind to the cells. In their 2012 paper, Langer et al. explain how they determined what cells are actually the target of anthrax toxin is in humans. The results that they obtained show that human alveolar epithelial cells (AECs) express the anthrax toxin receptor, and they are, therefore, the target cell in the human respiratory system (Langer).</p> | ||
<p>The experiment which they conducted revealed that AECs in human lungs express the ATR protein, allowing PA molecules to bind to those cells and transport lethal toxin into the cytosol of the cells. Also, once infected, these cells exhibit reduced barrier function, junction formation, and surfactant production. This reduced surfactant production is illustrated in Figure 2. These symptoms are all signs of acute lung injury caused by the anthrax toxin. The team suspected that these changes in the AECs allow for greater dissemination of B. anthracis from the lungs into the blood in the early stages of infection, and in later stages of infection, they cause severe edema in the pulmonary tissue (Langer).</p> | <p>The experiment which they conducted revealed that AECs in human lungs express the ATR protein, allowing PA molecules to bind to those cells and transport lethal toxin into the cytosol of the cells. Also, once infected, these cells exhibit reduced barrier function, junction formation, and surfactant production. This reduced surfactant production is illustrated in Figure 2. These symptoms are all signs of acute lung injury caused by the anthrax toxin. The team suspected that these changes in the AECs allow for greater dissemination of B. anthracis from the lungs into the blood in the early stages of infection, and in later stages of infection, they cause severe edema in the pulmonary tissue (Langer).</p> | ||
| Line 90: | Line 86: | ||
[Sample reference] [http://ijs.sgmjournals.org/cgi/reprint/50/2/489 Takai, K., Sugai, A., Itoh, T., and Horikoshi, K. "''Palaeococcus ferrophilus'' gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney". ''International Journal of Systematic and Evolutionary Microbiology''. 2000. Volume 50. p. 489-500.] | [Sample reference] [http://ijs.sgmjournals.org/cgi/reprint/50/2/489 Takai, K., Sugai, A., Itoh, T., and Horikoshi, K. "''Palaeococcus ferrophilus'' gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney". ''International Journal of Systematic and Evolutionary Microbiology''. 2000. Volume 50. p. 489-500.] | ||
Baillie, L., & Read, T. D. (2001). | <p>Baillie, L., & Read, T. D. (2001). Bacillus anthracis, a bug with attitude! Current Opinion in Microbiology, 4(1), 78-81. doi:10.1016/S1369-5274(00)00168-5 </p> | ||
Chung, M., Narayanan, A., Popova, T. G., Kashanchi, F., Bailey, C. L., & Popov, S. G. (2013). Bacillus anthracis-derived nitric oxide induces protein S-nitrosylation contributing to macrophage death. Biochemical and Biophysical Research Communications, 430(1), 125-130. doi:10.1016/j.bbrc.2012.11.042 | <p>Chung, M., Narayanan, A., Popova, T. G., Kashanchi, F., Bailey, C. L., & Popov, S. G. (2013). Bacillus anthracis-derived nitric oxide induces protein S-nitrosylation contributing to macrophage death. Biochemical and Biophysical Research Communications, 430(1), 125-130. doi:10.1016/j.bbrc.2012.11.042</p> | ||
Driks, A. (2009). The bacillus anthracis spore. Molecular Aspects of Medicine, 30(6), 368-373. doi:10.1016/j.mam.2009.08.001 | <p>Driks, A. (2009). The bacillus anthracis spore. Molecular Aspects of Medicine, 30(6), 368-373. doi:10.1016/j.mam.2009.08.001 </p> | ||
Grunow, R., Verbeek, L., Jacob, D., Holzmann, T., Birkenfeld, G., Wiens, D., . . . Reischl, U. (2012). Injection anthrax-a new outbreak in heroin users. Deutsches Arzteblatt International, 109(49), 843-848. doi:10.3238/arztebl.2012.0843 | <p>Grunow, R., Verbeek, L., Jacob, D., Holzmann, T., Birkenfeld, G., Wiens, D., . . . Reischl, U. (2012). Injection anthrax-a new outbreak in heroin users. Deutsches Arzteblatt International, 109(49), 843-848. doi:10.3238/arztebl.2012.0843 </p> | ||
Hanna, P. C., & Ireland, J. A. W. (1999). Understanding bacillus anthracis pathogenesis. Trends in Microbiology, 7(5), 180-182. doi:10.1016/S0966-842X(99)01507-3 | <p>Hanna, P. C., & Ireland, J. A. W. (1999). Understanding bacillus anthracis pathogenesis. Trends in Microbiology, 7(5), 180-182. doi:10.1016/S0966-842X(99)01507-3 </p> | ||
Langer, M., Duggan, E. S., Booth, J. L., & et al. (2012). Bacillus anthracis lethal toxin reduces human alveolar epithelial barrier function. Infection and Immunity, 80(12), 4374-4387. doi:10.1128/IAI.01011-12 | <p>Langer, M., Duggan, E. S., Booth, J. L., & et al. (2012). Bacillus anthracis lethal toxin reduces human alveolar epithelial barrier function. Infection and Immunity, 80(12), 4374-4387. doi:10.1128/IAI.01011-12 </p> | ||
Mechaly, A., Levy, H., Epstein, E., Rosenfeld, R., Marcus, H., Ben-Arie, E., . . . Mazor, O. (2012). A novel mechanism for antibody-based anthrax toxin neutralization: INHIBITION OF PREPORE-TO-PORE CONVERSION. Journal of Biological Chemistry, 287(39), 32665-32673. doi:10.1074/jbc.M112.400473 | <p>Mechaly, A., Levy, H., Epstein, E., Rosenfeld, R., Marcus, H., Ben-Arie, E., . . . Mazor, O. (2012). A novel mechanism for antibody-based anthrax toxin neutralization: INHIBITION OF PREPORE-TO-PORE CONVERSION. Journal of Biological Chemistry, 287(39), 32665-32673. doi:10.1074/jbc.M112.400473 </p> | ||
Mock, M., & Fouet, A. (2001). Anthrax. Annual Review of Microbiology, 55, 647-671. doi:10.1146/annurev.micro.55.1.647 | <p>Mock, M., & Fouet, A. (2001). Anthrax. Annual Review of Microbiology, 55, 647-671. doi:10.1146/annurev.micro.55.1.647 </p> | ||
Mock, M., & Mignot, T. (2003). Anthrax toxins and the host: A story of intimacy. Cellular Microbiology, 5(1), 15-23. doi:10.1046/j.1462-5822.2003.00253.x | <p>Mock, M., & Mignot, T. (2003). Anthrax toxins and the host: A story of intimacy. Cellular Microbiology, 5(1), 15-23. doi:10.1046/j.1462-5822.2003.00253.x </p> | ||
Rao, S. S., Mohan, K. V. K., & Atreya, C. D. (2010). Detection technologies for bacillus anthracis: Prospects and challenges. Journal of Microbiological Methods, 82(1), 1-10. doi:10.1016/j.mimet.2010.04.005 | <p>Rao, S. S., Mohan, K. V. K., & Atreya, C. D. (2010). Detection technologies for bacillus anthracis: Prospects and challenges. Journal of Microbiological Methods, 82(1), 1-10. doi:10.1016/j.mimet.2010.04.005 </p> | ||
Spencer, R. C. (2003). Bacillus anthracis. Journal of Clinical Pathology, 56(3), 182-187. | <p>Spencer, R. C. (2003). Bacillus anthracis. Journal of Clinical Pathology, 56(3), 182-187. </p> | ||
Revision as of 02:55, 25 April 2013
Introduction
By Connor Gibbons
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Anthrax is a disease that typically affects herbivores but can infect any mammal, including humans. It is caused by the bacterium Bacillus anthracis (B. anthracis). This disease has become a hot topic in today’s society, in which terrorism is becoming more prevalent, as it can be used in biological warfare. Recently, there has also been an increase in the number of cases of injection anthrax, a form of the disease that affects heroin users, in Europe (Grunow). However, this disease is not new. In fact, there is evidence of it throughout history. The fifth and sixth plagues in Egypt (Exodus, Chapter 9) have been attributed to this pathogen (Baillie).
The species derives its name from the Greek word anthrakis, meaning coal, because infection by the organism causes the formation of black, cutaneous eschars (Spencer). There are are three types of B. anthracis infection that are typically described: cutaneous anthrax (the cause of the black sores), inhalation anthrax (the most deadly form), and gastrointestinal anthrax (Mock, Mignot).
Organism
Bacillus anthracis, a member of the genus Bacillus, is a Gram-positive, rod-shaped bacteria that typically forms short chains of cells. It is an aerobic organism that is also capable of functioning as a facultative anaerobe. Bacillus anthracis is the only obligate pathogen in its genus, and a large reason that it is such a dangerous bacteria is the fact that it can produce spores that are resistant to very adverse environmental conditions such as heat, radiation, pressure, and chemical agents (Mock, “Anthrax”). These spores are able to live dormant in soil for decades (Spencer). Virulent strains of B. anthracis contain two large plasmids, pXO1 and pXO2. The first plasmid, pXO1, is 184.5 kbp in length and it contains the genes that encode the three secretory toxins produced by the bacteria. These toxins are lethal factor (LF), edema factor (EF), and protective antigen (PA). The second plasmid, pXO2, contains the genes capA, capB, and capC. These genes code for protein products that are involved in the synthesis of the polyglutamyl capsule, which functions to prevents phagocytosis of the active, vegetative form of B. anthracis. The loss of either of these plasmids typically results in at least a partial loss of virulence (Spencer). It has been observed, however, that high concentrations of the bacteria will still cause infection, even without the pXO2 plasmid. Further evidence shows that LF and EF work in tandem in the host organism to cause virulence, so EF- or LF-deficient mutants are not as effective at causing death or edema respectively (Mock, “Anthrax”).
Life Cycle
The Spore
When B. anthracis, in the vegetative form, enters the environment, leaving a dying host organism, sporulation occurs. Dormant endospores are the infectious particle of B. anthracis; infection only takes place when an endospore enters the body from the environment, whether through an abrasion on the skin of an organism, through ingestion, or through inhalation. Spores are ideal infections particles because, as mentioned earlier, they are extremely resistant to adverse environmental conditions (Mock, “Anthrax”). There have not been any observed cases of transmission of the disease between two living animals in the wild, because this would require the transfer of vegetative bacterial cells, not spores (Hanna).
Germination
When B. anthracis spores enter a host organism, they are phagocytosed by regional macrophages and transported to the lymph nodes (Rao). There, receptors on the inner membrane of the spore bind to molecules called germinants, which begins the germination process. The binding of the receptors causes rehydration of the spore and disintegration of the cortex and the coat (Driks), leaving the vegetative form of B. anthracis. Infection of the host organism proceeds until it is treated or the host dies, releasing the cells into the environment and starting the process over.
Pathogenesis
As noted above, infection by B. anthracis begins with the germination of a spore in the host organism. When germination occurs, the vegetative bacteria enter the blood stream and begin rapid extracellular multiplication. At this time, synthesis and extracellular secretion of capsule proteins and exotoxins also begins.
The exotoxins of B. anthracis act in binary combinations (LF + PA and EF + PA) to form lethal toxin (LeTx) and edema toxin (EdTx). Protective antigen molecules act by binding to a receptor on a target cell, inserting into the cell membrane, and translocating the bound toxin factor into the cytosol of the target cell (Langer). This is accomplished by the binding of PA to anthrax toxin receptor (ATR), a membrane protein located in many cell types. PA is then cleaved by a furin-family protease to reveal the LF and EF binding sites. PA then oligomerizes into a hepatomer and binds LF or EF, a competitive process between the two toxin factors. The newly formed LeTx or EdTx complexes are activated by acidic conditions in an endosome when they enter the cell via endocytosis, and they are then transferred into the cytosol.
Inside the cell, EF is an adenylate cyclase, which converts intracellular ATP into cAMP, a process that is dependent on the protein calmodulin, which is produced by the host cell and acts as a ligand for EF. This process causes a dramatic increase in intracellular cAMP, disrupting cell signalling as well as membrane permeability regulation, which leads to edema at and around the infection site (Chung). In the case of inhalation anthrax, this edema presents as pleural effusion. Lethal factor is a zinc metalloprotease that cleaves the N-terminus of mitogen-activated protein kinases (MAPKKs), which are involved in cell signaling pathways (Mock, Mignot). This process is illustrated in Figure 2. The exact physiological mechanisms by which LeTx and EdTx kill the organism are not yet known.
Clinical Symptoms and Diagnosis
Cutaneous Anthrax
Cutaneous anthrax infection occurs when B. anthracis spores enter the host via an opening in the skin, such as an abrasion, cut, or insect bite. In the two to three days post-infection, a small pimple-like papule forms. During the next 24 hours , vesicles form, making a raised ring around the papule, which ulcerates at this time. This ulcer dries out, forming a painless, black eschar, from which B. anthracis gets its name. Edema will be present around the site as well. In an uncomplicated case of cutaneous anthrax, the bacteria will not spread beyond the lesion, but if the infection is left untreated, ~20% of patients will become septic, which is very likely fatal (Spencer).
Gastrointestinal Anthrax
Gastrointestinal anthrax occurs when a human or other animal ingests contaminated meat that contains B. anthracis spores. This type of infection is very rare, and outside of Asia and Africa, few if any cases have been reported. Clinicians divide cases of this type of infection into two categories: abdominal and oro-esophageal. Patients suffering from the abdominal form experience nausea, vomiting, anorexia, and fever. These symptoms quickly progress are followed by increasing acute abdominal pain, bloody diarrhea, septicemia, and death. The oro-esophageal form of gastrointestinal anthrax presents with patients symptoms including sore throat, dysphagia, fever, cervical lymphadenopathy (enlargement of the lymph nodes in the neck), and edema.
Gastrointestinal anthrax has a high rate of mortality even though it is curable if caught early. This can be attributed to the non-specific symptoms, which resemble those of a severe case of influenza (Spencer).
Inhalation Anthrax
Resulting from the inhalation of B. anthracis spores, inhalation anthrax (also called respiratory anthrax) is mainly associated with industrial exposure in the textile or tanning industries. The onset of symptoms usually occurs two to five days post-exposure. These symptoms are usually non-specific and “flu-like” (mild fever, fatigue, mild cough, etc.). This stage of the infection lasts about two days abruptly ends with the onset of acute symptoms including trouble breathing, fever, rapid breathing, tachycardia, cyanosis, and pleural effusion. These symptoms continue to worsen until they eventually lead to coma and death. Meningitis, or swelling of the membranes surrounding the brain and spinal cord, also occurs in about 50% of patients who contract this form of the disease (Spencer).
Diagnosis
Clinical diagnosis of anthrax is performed by first culturing the skin lesion, blood, or cerebrospinal fluid and Gram staining the culture. The disease is indicated by the visualization of large Gram-positive bacilli forming short chains (Spencer).
Anthrax Toxin Neutralization with Antibody
During infection by B. anthracis, protective antigen is integral in the intoxication of the host cells by anthrax toxins. This protein, therefore, is a logical target for scientists seeking to neutralize the anthrax toxins. Mechaly, et al. have recently found that the antibody cAb29, does just that: it targets PA molecules, interfering with the function of the molecule and thereby neutralizing the anthrax toxin (Mechaly).
The Mechaly group, in attempting to determine the mechanism by which cAb29 functions to neutralize the toxins, found that cAb29 does not have any effect on the initial steps of the intoxication process: PA molecules are still able to bind to the target cell via receptors on the target cell membrane. These molecules are still cleaved by the furin-family protease and they still oligomerize into hepatomers which bind EF and LF molecules. Instead, they discovered that the antibody binds to the prepore, which is the complex of PA and either LF or EF before it is internalized by the cell, preventing the acid catalyzed transition to the transmembranal pore (Mechaly).
Their tests showed 100% survival of mice injected intravenously with cAb29 12 hours after initial exposure to B. anthracis (Figure 1). This is a very important discovery as it could possibly lead to a cure for the disease even if it is not caught in the very early stages of infection (Mechaly).
Target Cells in Humans in Inhalation Anthrax
Until recently, scientists believed that alveolar macrophages were the target cells for inhaled B. anthracis spores in humans because the disease had been previously shown to inhibit mouse alveolar macrophages. However, more recent studies have determined that human alveolar macrophages do not express the anthrax toxin receptor protein and are therefore are unaffected by anthrax toxins because PA molecules are unable to bind to the cells. In their 2012 paper, Langer et al. explain how they determined what cells are actually the target of anthrax toxin is in humans. The results that they obtained show that human alveolar epithelial cells (AECs) express the anthrax toxin receptor, and they are, therefore, the target cell in the human respiratory system (Langer).
The experiment which they conducted revealed that AECs in human lungs express the ATR protein, allowing PA molecules to bind to those cells and transport lethal toxin into the cytosol of the cells. Also, once infected, these cells exhibit reduced barrier function, junction formation, and surfactant production. This reduced surfactant production is illustrated in Figure 2. These symptoms are all signs of acute lung injury caused by the anthrax toxin. The team suspected that these changes in the AECs allow for greater dissemination of B. anthracis from the lungs into the blood in the early stages of infection, and in later stages of infection, they cause severe edema in the pulmonary tissue (Langer).
References
Baillie, L., & Read, T. D. (2001). Bacillus anthracis, a bug with attitude! Current Opinion in Microbiology, 4(1), 78-81. doi:10.1016/S1369-5274(00)00168-5
Chung, M., Narayanan, A., Popova, T. G., Kashanchi, F., Bailey, C. L., & Popov, S. G. (2013). Bacillus anthracis-derived nitric oxide induces protein S-nitrosylation contributing to macrophage death. Biochemical and Biophysical Research Communications, 430(1), 125-130. doi:10.1016/j.bbrc.2012.11.042
Driks, A. (2009). The bacillus anthracis spore. Molecular Aspects of Medicine, 30(6), 368-373. doi:10.1016/j.mam.2009.08.001
Grunow, R., Verbeek, L., Jacob, D., Holzmann, T., Birkenfeld, G., Wiens, D., . . . Reischl, U. (2012). Injection anthrax-a new outbreak in heroin users. Deutsches Arzteblatt International, 109(49), 843-848. doi:10.3238/arztebl.2012.0843
Hanna, P. C., & Ireland, J. A. W. (1999). Understanding bacillus anthracis pathogenesis. Trends in Microbiology, 7(5), 180-182. doi:10.1016/S0966-842X(99)01507-3
Langer, M., Duggan, E. S., Booth, J. L., & et al. (2012). Bacillus anthracis lethal toxin reduces human alveolar epithelial barrier function. Infection and Immunity, 80(12), 4374-4387. doi:10.1128/IAI.01011-12
Mechaly, A., Levy, H., Epstein, E., Rosenfeld, R., Marcus, H., Ben-Arie, E., . . . Mazor, O. (2012). A novel mechanism for antibody-based anthrax toxin neutralization: INHIBITION OF PREPORE-TO-PORE CONVERSION. Journal of Biological Chemistry, 287(39), 32665-32673. doi:10.1074/jbc.M112.400473
Mock, M., & Fouet, A. (2001). Anthrax. Annual Review of Microbiology, 55, 647-671. doi:10.1146/annurev.micro.55.1.647
Mock, M., & Mignot, T. (2003). Anthrax toxins and the host: A story of intimacy. Cellular Microbiology, 5(1), 15-23. doi:10.1046/j.1462-5822.2003.00253.x
Rao, S. S., Mohan, K. V. K., & Atreya, C. D. (2010). Detection technologies for bacillus anthracis: Prospects and challenges. Journal of Microbiological Methods, 82(1), 1-10. doi:10.1016/j.mimet.2010.04.005
Spencer, R. C. (2003). Bacillus anthracis. Journal of Clinical Pathology, 56(3), 182-187.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.