Bacterial Transcription Factors against Reactive Oxygen Species: Difference between revisions
| Line 27: | Line 27: | ||
===<br>Defensive strategy for Gram-positive bacteria: PerR=== | ===<br>Defensive strategy for Gram-positive bacteria: PerR=== | ||
====<br>H<sub>2</sub>O<sub>2</sub> Sensory mechanism of PerR==== | ====<br>H<sub>2</sub>O<sub>2</sub> Sensory mechanism of PerR==== | ||
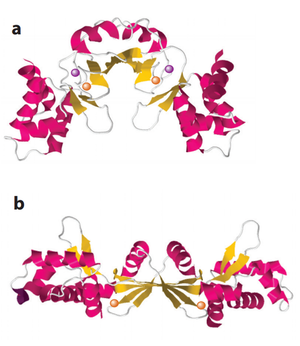
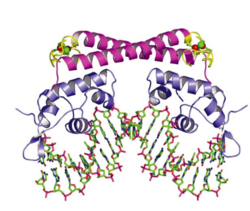
PerR is the common H<sub>2</sub>O<sub>2</sub>-inducible transcription factor in many Gram-positive bacteria such as <i>Bacillus subtilis</i> and <i>Staphylococcus aureus</i>. It belongs to the Fur protein family and uses metal oxidation reaction to sense H<sub>2</sub>O<sub>2</sub>. PerR is a dimer and each subunit has a structural zinc-binding site that irreversibly binds to Zn<sup>2+</sup>. Each subunit also contains at least one regulatory metal binding site. The metal in the regulatory site varies from organisms, usually Fe<sup>2+</sup> and/or Mn<sup>2+</sup>. <ref>[http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2958.2006.05313.x/full Traoré et al. (2006): Crystal structure of the apo‐PerR‐Zn protein from <i>Bacillus subtilis</i>. Molecular microbiology 61(5): 1211-1219.]</ref> <ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847443/ Jacquamet et al. (2009): Structural characterization of the active form of PerR: insights into the metal‐induced activation of PerR and Fur proteins for DNA binding. Molecular microbiology 73(1): 20-31.]</ref> When cellular H<sub>2</sub>O<sub>2</sub> concentration increases, it will oxidize the regulatory metal. Contrast to cysteine thiol-dependent OxyR, the oxidized metal cause rapid oxidation of histidine residues nearby and leads to a conformational change of the protein. <ref>[http://www.jbc.org/content/281/33/23567.long Lee & Helmann (2006): Biochemical characterization of the structural Zn<sup>2+</sup> site in the <i>Bacillus subtilis</i> peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.]</ref> This conformational change causes a decreased binding affinity of PerR to DNA. Since PerR usually functions as a repressor, its detachment from DNA relieves the inhibition on the gene expression of the regulon members. | PerR is the common H<sub>2</sub>O<sub>2</sub>-inducible transcription factor in many Gram-positive bacteria such as <i>Bacillus subtilis</i> and <i>Staphylococcus aureus</i>. It belongs to the Fur protein family and uses metal oxidation reaction to sense H<sub>2</sub>O<sub>2</sub>. PerR is a dimer and each subunit has a structural zinc-binding site that irreversibly binds to Zn<sup>2+</sup>. Each subunit also contains at least one regulatory metal binding site. The metal in the regulatory site varies from organisms, usually Fe<sup>2+</sup> and/or Mn<sup>2+</sup>. <ref>[http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2958.2006.05313.x/full Traoré et al. (2006): Crystal structure of the apo‐PerR‐Zn protein from <i>Bacillus subtilis</i>. Molecular microbiology 61(5): 1211-1219.]</ref> <ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847443/ Jacquamet et al. (2009): Structural characterization of the active form of PerR: insights into the metal‐induced activation of PerR and Fur proteins for DNA binding. Molecular microbiology 73(1): 20-31.]</ref> When cellular H<sub>2</sub>O<sub>2</sub> concentration increases, it will oxidize the regulatory metal. Contrast to cysteine thiol-dependent OxyR, the oxidized metal cause rapid oxidation of histidine residues nearby and leads to a conformational change of the protein. <ref>[http://www.jbc.org/content/281/33/23567.long Lee & Helmann (2006): Biochemical characterization of the structural Zn<sup>2+</sup> site in the <i>Bacillus subtilis</i> peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.]</ref> This conformational change causes a decreased binding affinity of PerR to DNA. Since PerR usually functions as a repressor, its detachment from DNA relieves the inhibition on the gene expression of the regulon members. [[Image:PerR.png|thumb|300px|right|Structure of PerR in <i>Bacillus subtilis</i>. (a) holoenzyme (b) demetallated (Manganese removed) enzyme. The orange balls are Zn and the purple ones are Mn]] | ||
====<br>PerR in <i>Bacillus subtilis</i>==== | ====<br>PerR in <i>Bacillus subtilis</i>==== | ||
PerR in <i>B. subtilis</i> (PerR<sub>BS</sub>) is the most studied PerR in bacteria. In <i>B. subtilis</i>, the metal in the regulatory site is Fe<sup>2+</sup>. PerR<sub>BS</sub> may also bind Mn<sup>2+</sup> as an alternative, which, however, as mentioned before, is not sensitive to H<sub>2</sub>O<sub>2</sub>. <ref>[http://www.pnas.org/content/92/18/8190.full.pdf Chen et al.(1995): Coordinate regulation of <i>Bacillus subtilis</i> peroxide stress genes by hydrogen peroxide and metal ions. Proceedings of the National Academy of Sciences 92(18): 8190-8194.]</ref> PerR<sub>BS</sub> shows same catalytic rate with H<sub>2</sub>O<sub>2</sub> as that of OxyR in <i>E. coli</i>. <ref>[http://www.jbc.org/content/281/33/23567.long Lee & Helmann (2006): Biochemical characterization of the structural Zn<sup>2+</sup> site in the <i>Bacillus subtilis</i> peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.]</ref> Compared with the reversible cysteine oxidation in OxyR, the oxidation of histidine residues in PerR<sub>BS</sub> seems to be irreversible, since there is no oxo-histidine reduction mechanism discovered yet | PerR in <i>B. subtilis</i> (PerR<sub>BS</sub>) is the most studied PerR in bacteria. In <i>B. subtilis</i>, the metal in the regulatory site is Fe<sup>2+</sup>. PerR<sub>BS</sub> may also bind Mn<sup>2+</sup> as an alternative, which, however, as mentioned before, is not sensitive to H<sub>2</sub>O<sub>2</sub>. <ref>[http://www.pnas.org/content/92/18/8190.full.pdf Chen et al.(1995): Coordinate regulation of <i>Bacillus subtilis</i> peroxide stress genes by hydrogen peroxide and metal ions. Proceedings of the National Academy of Sciences 92(18): 8190-8194.]</ref> PerR<sub>BS</sub> shows same catalytic rate with H<sub>2</sub>O<sub>2</sub> as that of OxyR in <i>E. coli</i>. <ref>[http://www.jbc.org/content/281/33/23567.long Lee & Helmann (2006): Biochemical characterization of the structural Zn<sup>2+</sup> site in the <i>Bacillus subtilis</i> peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.]</ref> Compared with the reversible cysteine oxidation in OxyR, the oxidation of histidine residues in PerR<sub>BS</sub> seems to be irreversible, since there is no oxo-histidine reduction mechanism discovered yet. The PerR<sub>BS</sub> regulon overlaps a lot with that of OxyR in Gram-negative bacteria, such as KatA, a KatG homolog, MrgA, a Dps homolog, and a Fur protein. <ref>[http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1998.00921.x/epdf Bsat et al.(1998): <i>Bacillus subtilis</i> contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Molecular microbiology 29(1): 189-198.]</ref> However, contrary to OxyR, PerR<sub>BS</sub> regulon does not include the enzymes related to maintain thiol and to reduce disulfide bonds.<ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4618077/ Imlay, J.A. (2015): Transcription factors that defend bacteria against reactive oxygen species 2015. Annual Review of Microbiology 69:93–108.]</ref> | ||
<br> | <br> | ||
====PerR in <i>Staphylococcus aureus</I>==== | ====PerR in <i>Staphylococcus aureus</I>==== | ||
<i>Staphylococcus aureus</i> is a Gram-positive pathogenic bacterium. As an opportunistic pathogen, it is often found on normal skin microflora and in the nasal passage and respiratory tract. However, on susceptible individuals, it is the most common cause of mild skin infections as well as often-fatal infections including sepsis and toxic shock syndrome (Lowy, 1998). Current therapeutics of <i>S. aureus</i> infection is increasingly complicated because of the emergence and prevalence of methicillin-resistant <i>S. aureus</i> (MRSA) across the world (Klevens et al., 2007). It has been known that in the infected organisms, their neutrophils use phagosome containing ROS to defend against <i>S. aureus</i> (Reviewd by Spaan et al., 2013; van Kessel et al., 2014). Therefore, in order to develop enhanced treatment to <i>S. aureus</i> infection, it is important to characterize the mechanism(s) that <i>S. aureus</i> uses to defend oxidative stress. | <i>Staphylococcus aureus</i> is a Gram-positive pathogenic bacterium. As an opportunistic pathogen, it is often found on normal skin microflora and in the nasal passage and respiratory tract. However, on susceptible individuals, it is the most common cause of mild skin infections as well as often-fatal infections including sepsis and toxic shock syndrome (Lowy, 1998). Current therapeutics of <i>S. aureus</i> infection is increasingly complicated because of the emergence and prevalence of methicillin-resistant <i>S. aureus</i> (MRSA) across the world (Klevens et al., 2007). It has been known that in the infected organisms, their neutrophils use phagosome containing ROS to defend against <i>S. aureus</i> (Reviewd by Spaan et al., 2013; van Kessel et al., 2014). Therefore, in order to develop enhanced treatment to <i>S. aureus</i> infection, it is important to characterize the mechanism(s) that <i>S. aureus</i> uses to defend oxidative stress. | ||
<br>PerR has been previously identified as the major transcription factor on <i>S. aureus</i> to response to oxidative burst (Horsburge et al., 2001a; Horsburge et al., 2001b). The characterization of PerR in <i>S. aureus</i> (PerR<sub>SA</sub>) has shown that it is a H<sub>2</sub>O<sub>2</sub>-inducible translation factor using the same sensory mechanism as <i>B. subtilis</i>. Together with PerR found in other bacteria, Ji et al. suggest that PerR and PerR-type transcription factors may be a conserved mechanism for Gram-positive bacteria against H<sub>2</sub>O<sub>2</sub> pressure (2015). However, under the same H<sub>2</sub>O<sub>2</sub> pressure, the expression levels of PerR regulon were higher in <i>S. aureus</i> expressing PerR<sub>SA</sub> than those expressing PerR<sub>BS</sub>, which suggests that <br>PerR<sub>SA</sub> was more sensitive to H<sub>2</sub>O<sub>2</sub> oxidation and thus more resistant to H<sub>2</sub>O<sub>2</sub>. | <br>PerR has been previously identified as the major transcription factor on <i>S. aureus</i> to response to oxidative burst (Horsburge et al., 2001a; Horsburge et al., 2001b). The characterization of PerR in <i>S. aureus</i> (PerR<sub>SA</sub>) has shown that it is a H<sub>2</sub>O<sub>2</sub>-inducible translation factor using the same sensory mechanism as <i>B. subtilis</i>. Together with PerR found in other bacteria, Ji et al. suggest that PerR and PerR-type transcription factors may be a conserved mechanism for Gram-positive bacteria against H<sub>2</sub>O<sub>2</sub> pressure (2015). However, under the same H<sub>2</sub>O<sub>2</sub> pressure, the expression levels of PerR regulon were higher in <i>S. aureus</i> expressing PerR<sub>SA</sub> than those expressing PerR<sub>BS</sub>, which suggests that <br>PerR<sub>SA</sub> was more sensitive to H<sub>2</sub>O<sub>2</sub> oxidation and thus more resistant to H<sub>2</sub>O<sub>2</sub>. | ||
Furthermore, Ji et al. used <i>Caenorhabditis elegans</i> as the model to study the relationship between H<sub>2</sub>O<sub>2</sub> sensitivity of PerR and pathogenesis of <i>S. aureus</i>. They observed that <i>S. aureus</i> expressing PerR<sub>BS</sub>killed <i>C. elegans</i> faster than PerR<sub>SA</sub>. This suggested that lower susceptibility of PerR to H<sub>2</sub>O<sub>2</sub> oxidation actually increases the virulence of <i>S. aureus</i>. | Furthermore, Ji et al. used <i>Caenorhabditis elegans</i> as the model to study the relationship between H<sub>2</sub>O<sub>2</sub> sensitivity of PerR and pathogenesis of <i>S. aureus</i>. They observed that <i>S. aureus</i> expressing PerR<sub>BS</sub> killed <i>C. elegans</i> faster than PerR<sub>SA</sub>. This suggested that lower susceptibility of PerR to H<sub>2</sub>O<sub>2</sub> oxidation actually increases the virulence of <i>S. aureus</i>. | ||
<br> | <br> | ||
<br> | <br> | ||
Revision as of 00:28, 27 April 2016
Overview
By Jiayu Chen
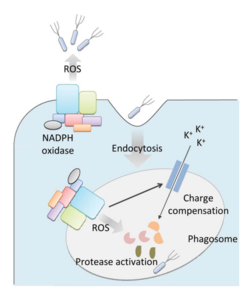
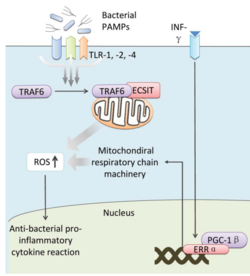
Bacteria live in a world full of toxic reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide (O2-), and hydroxyl radicals. ROS can cause severe damage to all kinds of cell components, including nucleic acids, lipids and proteins [1]. These highly reactive species are generated endogenously by accidental autoxidation of flavoproteins, a group of non-respiratory redox enzymes. [2] Thus, aerobic bacteria have evolved to maintain high concentration of enzymes that keep intracellular ROS below a lethal level. However, various conditions are known to increase these species above such threshold, inflicting oxidative stress to the bacteria, a phenomenon called “oxidative burst.” [3] One of the major scenario is when bacteria encounter ROS generated by the immune system of their host as a defensive weapon. In human, phagocytes including neutrophils use NADPH oxidase 2 (NOX2) complex as one of the major enzyme mediating the immune response by generating antimicrobial ROS. The resultant ROS would be secreted either outside of the cell as a trap to kill bacteria, or into phagolysosome where internalized bacteria would be degraded. Another situation that may happen during bacterial infection is the ROS generated by the mitochondria. Such defensive mechanism is mediated by many signaling molecule in the Toll pathways including several Toll-like receptors (TLRs), tumor necrosis factor receptor-associated factor 6 (TRAF6) and evolutionarily conserved signaling intermediate in Toll pathways (ECSIT).
The activation of Toll pathways resulted in increased cellular ROS as well as anti-bacterial pro-inflammatory cytokine reaction in the nucleus. Pro-inflammatory Th1 cytokine IFN-γ is also involved as a transcriptional regulator via activation of estrogen-related receptor α (ERRα) and PPARγ-coactivator-1β (PGC-1β) in the nucleus. Such interaction induces the genes that encode mitochondrial respiratory chain machinery and thus increase cellular ROS concentration. [4]
Therefore, bacteria have developed defensive systems to deal with such situations. One mechanism is ROS inducible transcriptional factors that regulate gene expression of a variety of enzymes to respond to the oxidative stress. Three redox-sensitive transcription factors in bacteria have been intensively studied: OxyR and PerR against H2O2, and SoxR against O2- [5].
OxyR is induced by H2O2 and activated by reversible disulfide bond formation in its active site. [6] OxyR is widely distributed in Gram-negative bacteria, such as E. coli. The binding of OxyR to DNA generally turns on the gene expression of a large range of oxidative stress defense enzymes. [7] Recent research also has shown that Gram-negative bacteria Vibrio vulnificus, facultative aerobes, contain two OxyR-type regulators with similar sensory mechanism but different sensitivity in order to fine-tune the expression of genes against H2O2 pressure.[8]
PerR is the equivalent H2O2 sensor protein in many Gram-positive bacteria, such as Staphylococcus aureus and Bacillus subtilis. When PerR is in its reduced form, its binding to DNA generally serves as an inhibitor of the expression of target genes. PerR is activated by oxidation of its regulatory metal, either Fe2+ or Mn2+, which leads to conformational change in the protein and dissociation from DNA, thus relieving the inhibition and initiating transcription of H2O2 defensive genes. [9] [10]
SoxR is conserved widely in both proteobacteria and actinobacteria with an O2- sensitive metal complex in its active site. In E. coli, The binding of SoxR to DNA induces the transcription of a secondary transcription factor, SoxS, which activates a group of enzymes suppressing the level of O2-. [11] [12] [13]
Defending against hydrogen peroxide (H2O2)
Why is H2O2 poisonous?
Environmental H2O2 is primarily generated as a metabolism by-product and as defense mechanism of plants, animals and bacteria to other microbes. [14] [15] [16] Because its chemical structure is similar to that of water and has no charge, H2O2 can easily penetrate the membrane. Once it enters the cell, H2O2 damages the cell components through the Fenton Reaction in which H2O2 oxidizes Fe2+ ion, generating one Fe3+ ion and one hydroxyl radical. Free hydroxyl radicals can further react with lipids, nucleic acids and the disulfide bonds in proteins, which are all critical cellular components. [17] For example, guanine is one of the most vulnerable targets to hydroxyl radical because of its small reduction potential. The resulting product is often highly mutagenic 8-hydroxyguanine, because it is able to base pair with adenine such a way that allows it to escape from the DNA mismatch detection system. [18] The Fenton reaction also impedes the function of Fe2+-dependent enzymes that are distributed in various cellular processes.[19]
Defensive strategy for Gram-negative bacteria: OxyR
H2O2 sensory mechanism of OxyR
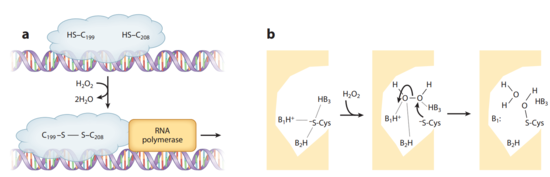
Crystal structures of OxyR in different bacteria have shown that it is a tetrameric protein. Each monomer contains a C-terminal regulatory domain and an N-terminal DNA-binding domain.The redox-switch mechanism in OxyR depends on two H2O2 sensory cysteine residues (Cys-199 and Cys-208) in its C-terminal. Cys-199 residue locates in a hydrophobic pocket, where it is stabilized by hydrophobic interaction of nearby Leucine residues. Cys-199 is rapidly oxidized by H2O2 and forms in a sulfenic acid intermediate (-SOH). This intermediate is not stable in the hydrophobic pocket, and thus lead to a conformational change that flips out the oxidized Cys-199. A flexible amino acid region between these two Cysteine residues then brings Cys-199 to the proximity of the Cys-208 to form a disulfide bond. This bond formation induces OxyR conformational change in its regulatory domain, recruiting RNA polymerase and thus stimulating the expression of genes against H2O2 stress.[20] [21] Furthermore, the interaction between OxyR and RNA polymerase seems to be able to reduce the disulfide bond and makes the transcriptional regulation reversible.[22]
OxyR in E. coli
As the model organism in microbiology study, the OxyR of E. colihas been extensively studied in past decades. A genome-wide transcription profile of E. coli using DNA microarray has shown that 23 genes are significantly induced (>20-fold) under H2O2 stress. [23] KatG, a catalase, and AphC, a NADH peroxidase, are activated, both of which directly reduce cellular peroxide. [24] [25] Several iron-related enzymes are also induced. Dps, a ferritin homolog, is most significantly upregulated (180-fold). Dps oxidizes cellular free Fe2+, thus inhibits the Fenton chemistry and protect DNA from hydroxyl radical damage. [26] [27] [28] Similarly, in order to decrease free cellular Fe2+ concentration, a Ferric uptake regulator (Fur) protein is also induced, which serves as an inhibitor to iron import. Furthermore, MntH, a Mn importer, is induced, too. Mn2+ cannot be oxidized by H2O2and is able to serve as enzymatic cofactor. Thus, higher cellular Mn2+ concentration allows Mn2+ to substitute for Fe2+ and protect the iron-dependent enzymes from damage cause by the Fenton chemistry. [29] There are other regulon members that are induced significantly under H2O2 pressure. However, the functions of some are still unknown, while the others’ roles seem to be less relevant after mutation assay. Notably, there is no direct DNA or lipid repair gene regulated by OxyR.
OxyRs in Vibrio vulnificus
Vibrio vulnificus is a pathogenic Gram-negative bacterium. Although the number of infected individual is relatively low compared with other member in the Vibrio genus, it could result in severe and usually fatal infections on immunocompromised individuals. The infection of V. vulnificus is usually related to consumption of raw shellfish, which engross the bacteria via filter feeding. V. vulnificus can lead to two kinds of diseases: septicemia and tissue necrosis, both of which develop very quickly and could lead to fatality. [30] Since immune system defends against this kind of pathogenic bacteria by generating ROS, thorough characterization of the mechanism of V. vulnificus under such stress is crucial to develop better clinical treatment to its infection. [31]
In previous research, two distinct peroxiredoxins (Prx), Prx1 and Prx2, have been identified in gram-negative facultative aerobe V. vulnificus [32] Prx1 is previously known as the homolog of NADH peroxidase AhpC in V. vulnificus. Prx1 is regulated by transcription factor OxyR in V. vulnificus and has similar function as that found in E. coli. [33] [34] However, the discovery and characterization of Prx2 led researchers to hypothesize the presence of another OxyR-type transcription factor in V. vulnificus. Prx2 shares low amino acid sequence identity with Prx1 and other bacterial AhpC. Prx2 is able to sense H2O2 at a level that is much lower than what Prx1 is able to do. Since V. vulnificus is facultative aerobic, two Prxs thus allow the bacteria to optimize the defendant process against different amount of H2O2. However, it is not clear how V. vulnificus regulate the gene expression of the second Prx.
Kim et al. successfully identified and characterized OxyR2, a new OxyR-type transcription factor in V. vulnificus. Sequence analysis shows that OxyR2 shares 34% amino acid sequence identity to the first OxyR (thereafter OxyR1) and contain the two Cysteine residues located corresponding to the redox-sensitive ones in OxyR1. Similarly, the two Cysteine residues in OxyR2 are subject to H2O2 oxidation to form a reversible disulfide bond. Prx2 expression is only induced by oxidized form of OxyR2 in vivo. The minimum concentration of H2O2 required to oxidize OxyR2 is much lower than that of OxyR1, which explains the high sensitivity of OxyR2 and thus that of Prx2. Compared to OxyR1, the enhanced H2O2 sensitivity could because of the fact that the microenvironment that the redox-active Cysteine residues locate are different. In OxyR1, Cys-199 is adjacent to a negatively charged Asp residue, while in OxyR2, the corresponding residue Cys-206 is adjacent to a positively charged Lys residue, which could enhance the nucleophilicity of Cys-206 by decreasing the thiol pKa, resulting in a higher reactivity to H2O2.
By possessing two OxyRs with different affinity to H2O2, V. vulnificus are able to fine-tune the detoxification of different amount of H2O2 they may encounter in natural environment. Such optimization could be an evolutionary advantage relevant to its pathogenesis. Furthermore, BLAST search reveals that there are many other bacteria containing homologs of both OxyR1 and OxyR2, suggesting that the coexistence of both OxyRs is widely distributed in bacteria.
From a medical perspective, mutation on the key Cysteine residues in OxyR2 resulted in lower concentration of cellular lactate dehydrogenase in the infected host cells, which indicated decreasing cytotoxicity caused by infection. In addition, during infection, the oxyR2 mutants showed decreased growth rate compared to the wild type. This could be explained by the fact that the lack of OxyR2 caused decreased Prx2 in the bacteria, so they were not able to fight against the ROS generated by the immune system of the host cells. These results implied that OxyR2 might be important for the virulence of V. vulnificus.[35]
Defensive strategy for Gram-positive bacteria: PerR
H2O2 Sensory mechanism of PerR
PerR is the common H2O2-inducible transcription factor in many Gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus. It belongs to the Fur protein family and uses metal oxidation reaction to sense H2O2. PerR is a dimer and each subunit has a structural zinc-binding site that irreversibly binds to Zn2+. Each subunit also contains at least one regulatory metal binding site. The metal in the regulatory site varies from organisms, usually Fe2+ and/or Mn2+. [36] [37] When cellular H2O2 concentration increases, it will oxidize the regulatory metal. Contrast to cysteine thiol-dependent OxyR, the oxidized metal cause rapid oxidation of histidine residues nearby and leads to a conformational change of the protein. [38] This conformational change causes a decreased binding affinity of PerR to DNA. Since PerR usually functions as a repressor, its detachment from DNA relieves the inhibition on the gene expression of the regulon members.
PerR in Bacillus subtilis
PerR in B. subtilis (PerRBS) is the most studied PerR in bacteria. In B. subtilis, the metal in the regulatory site is Fe2+. PerRBS may also bind Mn2+ as an alternative, which, however, as mentioned before, is not sensitive to H2O2. [39] PerRBS shows same catalytic rate with H2O2 as that of OxyR in E. coli. [40] Compared with the reversible cysteine oxidation in OxyR, the oxidation of histidine residues in PerRBS seems to be irreversible, since there is no oxo-histidine reduction mechanism discovered yet. The PerRBS regulon overlaps a lot with that of OxyR in Gram-negative bacteria, such as KatA, a KatG homolog, MrgA, a Dps homolog, and a Fur protein. [41] However, contrary to OxyR, PerRBS regulon does not include the enzymes related to maintain thiol and to reduce disulfide bonds.[42]
PerR in Staphylococcus aureus
Staphylococcus aureus is a Gram-positive pathogenic bacterium. As an opportunistic pathogen, it is often found on normal skin microflora and in the nasal passage and respiratory tract. However, on susceptible individuals, it is the most common cause of mild skin infections as well as often-fatal infections including sepsis and toxic shock syndrome (Lowy, 1998). Current therapeutics of S. aureus infection is increasingly complicated because of the emergence and prevalence of methicillin-resistant S. aureus (MRSA) across the world (Klevens et al., 2007). It has been known that in the infected organisms, their neutrophils use phagosome containing ROS to defend against S. aureus (Reviewd by Spaan et al., 2013; van Kessel et al., 2014). Therefore, in order to develop enhanced treatment to S. aureus infection, it is important to characterize the mechanism(s) that S. aureus uses to defend oxidative stress.
PerR has been previously identified as the major transcription factor on S. aureus to response to oxidative burst (Horsburge et al., 2001a; Horsburge et al., 2001b). The characterization of PerR in S. aureus (PerRSA) has shown that it is a H2O2-inducible translation factor using the same sensory mechanism as B. subtilis. Together with PerR found in other bacteria, Ji et al. suggest that PerR and PerR-type transcription factors may be a conserved mechanism for Gram-positive bacteria against H2O2 pressure (2015). However, under the same H2O2 pressure, the expression levels of PerR regulon were higher in S. aureus expressing PerRSA than those expressing PerRBS, which suggests that
PerRSA was more sensitive to H2O2 oxidation and thus more resistant to H2O2.
Furthermore, Ji et al. used Caenorhabditis elegans as the model to study the relationship between H2O2 sensitivity of PerR and pathogenesis of S. aureus. They observed that S. aureus expressing PerRBS killed C. elegans faster than PerRSA. This suggested that lower susceptibility of PerR to H2O2 oxidation actually increases the virulence of S. aureus.
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Halliwell (2006): Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiology 141(2):312–322.
- ↑ Seaver and Imlay (2004): Are Respiratory Enzymes the Primary Sources of Intracellular Hydrogen Peroxide? The Journal of Biological Chemistry, 279:48742-48750.
- ↑ Apostol et al. (1989): Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells: Role in Defense and Signal Transduction—Commentary. Plant Physiology, 90:109–116.
- ↑ Reviewed by Yang et al.(2013): Reactive oxygen species in the immune system. International Reviews of Immunology 32(3):249-70.
- ↑ Imlay, J.A. (2015): Transcription factors that defend bacteria against reactive oxygen species 2015. Annual Review of Microbiology 69:93–108.
- ↑ Choi et al. (2013): Structural Basis of the Redox Switch in the OxyR Transcription Factor. Cell 105(1):103-113.
- ↑ Zheng et al. (1998): DNA Microarray-Mediated Transcriptional Profiling of the Escherichia coli Response to Hydrogen Peroxide. Journal of Bacteriology 183(15): 4562-4570.
- ↑ Kim et al. (2014): Distinct characteristics of OxyR2, a new OxyR-type regulator, ensuring expression of Peroxiredoxin 2 detoxifying low levels of hydrogen peroxide in Vibrio vulnificus. Molecular Microbiology 93(5):992–1009.
- ↑ Ji et al.(2015): Staphylococcus aureus PerR Is a Hypersensitive Hydrogen Peroxide Sensor using Iron-mediated Histidine Oxidation. The Journal of Biological Chemistry 290:20374-20386.
- ↑ Lee & Helmann (2006): The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363-367.
- ↑ Greenberg et al. (1990): Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. PNAS 87: 6181-6185.
- ↑ Tsaneva and Weiss, (1990): soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. Journal of Bacteriology 172(8):4197-205.
- ↑ Blanchard et al. (2007): Rapid Changes in Gene Expression Dynamics in Response to Superoxide Reveal SoxRS-Dependent and Independent Transcriptional Networks. PLoS ONE 2(11): e1186.
- ↑ Mehdy, (1994): Active Oxygen Species in Plant Defense against Pathogens. Plant Physiology 105(2):467-472.
- ↑ Bedard and Krause (2007): The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological Reviews 87(1):245-313.
- ↑ Seki et al. (2004): Hydrogen Peroxide Production in Streptococcus pyogenes: Involvement of Lactate Oxidase and Coupling with Aerobic Utilization of Lactate. Journal of Bacteriology 186(7):2046–2051.
- ↑ Machlin and Bendich (1987): Free radical tissue damage: protective role of antioxidant nutrients. The FASEB Journal 1(6):441-445.
- ↑ Hogg et al. (2005): Bumps in the road: how replicative DNA polymerases see DNA damage. Current opinion in structural biology 15(1): 86-93.
- ↑ Imlay et al. (1988): Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240(4852): 640-642.
- ↑ Choi et al. (2013): Structural Basis of the Redox Switch in the OxyR Transcription Factor. Cell 105(1):103-113.
- ↑ Jo et al. (2014): Structural details of the OxyR peroxide-sensing mechanism. PNAS 112(20):6443–6448.
- ↑ Choi et al. (2013): Structural Basis of the Redox Switch in the OxyR Transcription Factor. Cell 105(1):103-113.
- ↑ Zheng et al. (1998): DNA Microarray-Mediated Transcriptional Profiling of the Escherichia coli Response to Hydrogen Peroxide. Journal of Bacteriology 183(15): 4562-4570.
- ↑ Heym et al.: Missense mutations in the catalase‐peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Molecular microbiology 15(2):235-245.
- ↑ Ellis & Poole (1997): Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry 36(43):13349-13356.
- ↑ Grant et al.(1998): The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nature Structural & Molecular Biology 5(4): 294-303.
- ↑ Ilari et al. (2005): The unusual intersubunit ferroxidase center of Listeria innocua Dps is required for hydrogen peroxide detoxification but not for iron uptake. A study with site-specific mutants. Biochemistry 44(15): 5579-5587.
- ↑ Chiancone & Ceci (2010): The multifaceted capacity of Dps proteins to combat bacterial stress conditions: detoxification of iron and hydrogen peroxide and DNA binding. Biochimica et Biophysica Acta (BBA)-General Subjects, 1800(8): 798-805.
- ↑ Kehres et al. (2000). The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Molecular microbiology 36(5): 1085-1100.
- ↑ Strom & Paranjpye (2000): Epidemiology and pathogenesis of Vibrio vulnificus. Microbes and infection 2(2): 177-188.
- ↑ Chung et al. (2010): RtxA1-induced expression of the small GTPase Rac2 plays a key role in the pathogenicity of Vibrio vulnificus. Journal of Infectious Diseases, 201(1): 97-105.
- ↑ Bang et al. (2012): Distinct characteristics of two 2-Cys peroxiredoxins of Vibrio vulnificus suggesting differential roles in detoxifying oxidative stress. Journal of Biological Chemistry 287(51): 42516-42524.
- ↑ Baek et al. (2009): Identification of the Vibrio vulnificus ahpCl gene and its influence on survival under oxidative stress and virulence. The Journal of Microbiology 47(5):624-632.
- ↑ Kong et al. (2004): Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS microbiology ecology 50(3):133-142.
- ↑ Kim et al. (2014): Distinct characteristics of OxyR2, a new OxyR-type regulator, ensuring expression of Peroxiredoxin 2 detoxifying low levels of hydrogen peroxide in Vibrio vulnificus. Molecular Microbiology 93(5):992–1009.
- ↑ Traoré et al. (2006): Crystal structure of the apo‐PerR‐Zn protein from Bacillus subtilis. Molecular microbiology 61(5): 1211-1219.
- ↑ Jacquamet et al. (2009): Structural characterization of the active form of PerR: insights into the metal‐induced activation of PerR and Fur proteins for DNA binding. Molecular microbiology 73(1): 20-31.
- ↑ Lee & Helmann (2006): Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.
- ↑ Chen et al.(1995): Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proceedings of the National Academy of Sciences 92(18): 8190-8194.
- ↑ Lee & Helmann (2006): Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. Journal of Biological Chemistry 281(33): 23567-23578.
- ↑ Bsat et al.(1998): Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Molecular microbiology 29(1): 189-198.
- ↑ Imlay, J.A. (2015): Transcription factors that defend bacteria against reactive oxygen species 2015. Annual Review of Microbiology 69:93–108.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.