Botryococcus braunii
Classification
Domain: Eukaryota
Division: Chlorophyta
Class: Trebouxiophyceae
Order: incertae sedis
Family: Botryococcaceae
Species
|
NCBI: Botryococcus braunii [1] |
Botryococcus braunii
Description and Significance
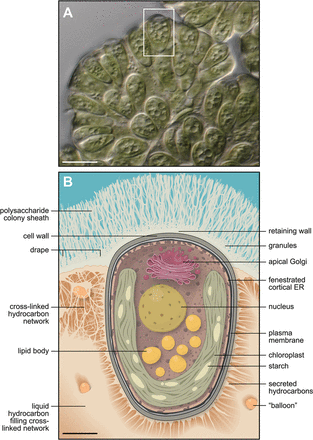
- Botryococcus braunii are unicellular, planktonic, oval-shaped algae. The individuals live in a biofilm community that helps contribute to their external hydrocarbon production 3. The extracellular matrix is a complex three component system that all works together to produce the external hydrocarbons as well as protect each cell 13. The biofilm is so strong so that the cells can withstand drying conditions as well as extreme temperatures for short amounts of time to allow dispersal to be easier for the organism. The outer layer of cells in the community contains a high number of chloroplasts.
- They mainly live in fresh to brackish oligotrophic bodies of water with low nutrient content. They thrive when the amount of dissolved inorganic phosphorus in the water increases. They can tolerate temperature from 40˚ C to -20˚ C for short amounts of times but prefer around 23˚ C 5. These are fully photosynthetic organisms so they require reasonable access to sunlight to carry out its metabolic activities.
Genome Structure
- Botryococcus braunii is a eukaryotic organism so it has linear DNA. The genome size of B. braunii depends on the race being examined. There are three races of Botryococcus: A, B, and L. They are classified based on the types of hydrocarbons they produce 1. Race A has a genome size of about 166.0±0.4 Mb (million bases). Race B has about 166.2±2.2 Mb. Race L has a larger genome size than its brothers at about 211.3±1.7 Mb 12.
- The codon usage of B. braunii was looked at for possible genetic engineering capabilities and showed that unlike most green algae species that have a high content of guanine and cytosine, B. braunii had a much lower number of G’s and C’s 11. The codon usage was closer to bacteria, mammals, and land plants. This means that B. braunii is likely to accept and use genetic material from non-microalgae species with little to no problems 7. After replicating genes from B. braunii, a 3’ (prime) untranslated region of about 1009 bp which is considerably larger than the 3’ UTR of other organisms. This feature most likely contributes to B. braunii’s ability to accept genetic material from non-microalgae organisms.
- Analysis of the genome of many strains of B. braunii show that they contain genes that code for squalene synthase and squalene synthase-like proteins that are especially important for the synthesis for the production of their oils. Interestingly, squalene is also a key substance that exists in the liver of sharks 12.
Cell Structure, Metabolism and Life Cycle
- The B. braunii organisms live in a community in the form of a complex biofilm matrix that is shaped like a cup that collects the hydrocarbons produced by each cell. A single-celled organism under the prime conditions will start secreting cellulose and pectin that forms the cell membrane of the original cup. After each division, every cell forms a new little pocket that folds inside the original cup all filled with hydrocarbons 4. The Extracellular matrix has a three component system: the individuals have a fibrous cell wall of their own that connects to all the other individuals with cross linked hydrocarbons. The whole biofilm is then covered with a saccharide sheath that the external hydrocarbons bubble out from 13. The outer layer of cells in the community contains a large quantity of chloroplasts and a small amount of starch. The cell wall of Botryococcus braunii is made up of biopolymers that help to distinguish between the three different races of B. braunii. The biopolymers are resistant to chemical degrading, not involving oxygen, like acetolysis. The resistive properties lie in very long fatty chain acid derivatives that come from oleic acid. The hydrocarbons produced that are able to be harvested for fuel come from the decarboxylation of these derivatives of oleic acid 2. The hydrocarbons it produces depends on the race of B. braunii. Most of them exist as Triterpenes. It does not require any metabolic starting conditions like nitrogen starvation. It accumulates hydrocarbon under all conditions, it just varies in the speed. The organism was tested to reduce the phosphorus and nitrogen in waste water and was found to produce more hydrocarbons at a 75% dilution 10.
Ecology and Pathogenesis
- As mentioned, B. braunii are cosmopolitan organisms that can live in a large range of conditions. They are mainly found in temperate freshwater with very low nutrient content. They can be found mainly in a strong biofilm matrix of only other B. braunii individuals. B. braunii are a major interest in the fuel production industry because 40-75% of their dry mass is made up of hydrocarbons that they produce in large amounts that resemble petroleum at a 90% efficiency rate. These provide raw materials that can be converted into fuel for vehicles like cars and jets 8. Using biodiesel from algae provides many environmentally friendly pluses such as it recycling large amounts of CO2 in the atmosphere for their photosynthetic metabolism, they use the land that is not fit for other biodiesel source crops, and they break down faster than petro-diesel 9. Algae biodiesel is a great alternative to the production of other biodiesels and the burning of the rest of our natural resources. Studies also support the notion that this organism and its ancient relatives are the reason for some of the petroleum reserves from the Ordovician period to now 1. The main problem with this alternative is the global demand of fuel is 90 million barrels a day, it just could not produce enough to fit the needs of the modern world 6.
- This microbe has no pathogenic properties.
References
1. Banerjee, A. (2002). Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Critical Reviews in Biotechnology, 22(3): p. 245-79. Retrieved April 16, 2014. PubMed Database.
2. Borowitzka, M.A., & Hoheimani, N.R. (2013). Algal Lipids and their Metabolism. Algae for Biofuels and Energy (p. 24). Dordrecht: Springer. Retrieved April 17, 2014.
3. Botryococcus braunii. (2014, May 4). Wikipedia. Retrieved April 16, 2014. Web. <http://en.wikipedia.org/wiki/Botryococcus_braunii>
4. Demetrescu, E. (1999). The Chlorococcalean Alga Botryococcus and Its Significance in Hydrocarbon Exploration: Basic Botryococcus Morphotypes and Related Hydrocarbon Constituents. National Institute of Marine Geology and Geo-ecology, 4: p. 159. Retrieved April 17, 2014. Web. GeoEcoMar.
5. Demura, M., Ioki, M., Kawachi, M., Nakajima, N., Watanabe, M.M. (2013). Desiccation tolerance of Botryococcus braunii (Trebouxiophyceae, Chlorophyta) and extreme temperature tolerance of dehydrated cells. Journal of Applied Phycology, 26: p. 49-53. Retrieved April 16, 2014. PubMed Database.
6. Madigan, M.T., Martinko, J.M., Stahl, D.A., & Clark, D.P. (2012). Commercial Products and Biotechnology. Brock Biology of Microorganisms (13th ed., p. 428). San Francisco, CA: Pearson as Benjamin Cummings.
7. Ioki, M., Baba, M., Nakajima, N., Shiraiwa, Y., Watanabe, M.M. (2013). Codon usage of Botryococcus braunii (Trebouxiophyceae, Chlorophyta): implications for genetic engineering applications. Phycologia, 52(4): p. 352-56. Retrieved April 16, 2014. Web. International Phycological Society.
8. Our Projects: Why sequence Botryococcus braunii? (2010). US Department of Energy: Joint Genome Institute. Retrieved April 13, 2014. Web. <http://jgi.doe.gov/why-sequence-botryococcus-braunii/>
9. Richardson, J.W., Outlaw, J.L., Allison, M. (2010). The Economics of Microalgae Oil. The Journal of Agrobiotechnology Management and Economics, 13(2): article 4. Retrieved April 17, 2014. Web. AgBioForum.
10. Seyhaneyildiz Can, S., Demir, V., Aslan Korkmaz, S., Can, E. (2013). Treatment of domestic waste water with Botryococcus braunii (Chlorophyceae). Journal of Food, Agriculture and the Environment, 11(3&4): p. 2128-30. Retrieved April 15, 2014. Web. WFL Publisher: Science and Technology.
11. Weiss, T.L., Johnston, J.S., Fujisawa, K., Sumimoto, K., Okada, S., Chappell, J., Devarenne, T.P. (2010). Phylogenetic placement, genome size, and GC content of the liquid-hydrocarbon producing green microalga Botryococcus braunii strain Berkeley (Showa)(Chlorophyta). Journal of Applied Phycology, 46: p. 534-40. Retrieved 17, 2014.
12. Weiss, T.L., Johnston, J.S., Fujisawa, K., Okada, S., Devarenne, T.P. (2010). Genome size and phylogenetic analysis of the A and L races of Botryococcus braunii. Journal of Applied Phycology, 23: p. 833-39. Retrieved April 17, 2014.
13. Weiss, T.L., Roth, R., Goodson, C., Vitha, S., Black, I., Azadi, P., Rusch, J., Holzenburg, A., Devarenne, T.P., Goodenough, U. (2012). Colony Organization in the Green Alga Botryococcus braunii (Race B) Is Specified by a Complex Extracellular Matrix. American Society for Microbiology: Eukaryotic Cell 11(12): p. 1424. Retrieved April 17, 2014. Web. American Society for Microbiology.
Author
Page authored by Justine Miller and Nicole Hansen, students of Dr. Edward Walker and Dr. Kazem Kashefi at Michigan State University.
