CCR5, Delta-32 Mutation and the HIV Infection Pathway
By Jon Funder Hansen
Introduction
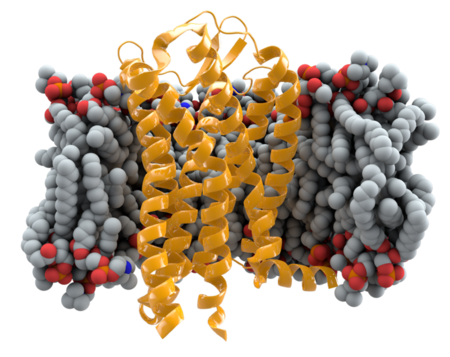
CCR5 (C-C chemokine receptor type 5) is surface protein located on the plasma membrane of white blood cells. CCR5 acts as a cytokine receptor and is primarily expressed on cells involved in the immune response, such as T-cells and macrophages. Individuals with mutations in one or both of the CCR5 alleles exhibit resistance to the Human Immunodeficiency Virus type 1 (HIV-1), the most common strain of the virus, as HIV-1 uses CCR5 as a co-receptor of infection.
Function
Chemokines are proteins that are involved in the direction of immune cell migration in the body. There are over fifty human chemokines that all work by binding to G-protein-coupled chemokine receptors, such as CCR5, on the surface of cells. Chemokines are grouped into two major families, inflammatory and homeostatic. CCR5 is one of the eighteen human chemokine receptors that have been identified so far. CCR5 regulates both the trafficking and effector molecule production of T-cells, immature dendritic cells and macrophages.[1]
CCR5 is one of three high affinity receptors of CCL5 (along with CCR1 and CCR3), which is an inflammatory chemokine that acts as one of the key regulators of T-cell inflammatory migration, directing the T-cells towards sites of infection.[2] Research suggest that CCL5:CCR5 Axis mediated signaling could serve an important role in mucosal Trypanosoma cruzi protection as well as B-cell activation. The CCR5-CCL5 Axis may be critical for the optimal control of T. cruzi replication.[3] It has been shown that CCR5-CCL5 interactions provide antiapoptotic signals that increase macrophage survival during mouse parainfluenza and human influenza infections in mice. The lack of the CCL5 chemokine in infected mice cause immunodeficiency, leading to excessive airway inflammation, delayed viral clearance and respiratory death. CCL5 protection requires the activation of the CCR5 receptor to stop cell to cell infections by macrophages.[4]
.
HIV Infection
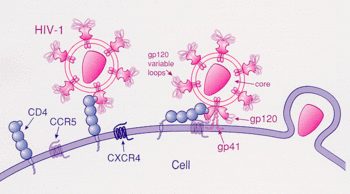
HIV-1 infection of a cell starts with the interaction between the envelope glycoprotein gp120 and the host leukocyte glycoprotein receptor (CD4), and is followed by the binding of the virus to either CCR5 or CXCR4. The high affinity between gp120 and CD4 and the subsequent binding with the CD4 protein, exposes the third variable region (V3) loop of the gp120 which allows it to bind with the chemokine receptor (CCR5 or CXCR4). The binding of the V3 loop with the chemokine receptor leads to a series of structural rearrangements in GP120 that leads to the fusion of the virus and cell membranes.[5]
CCR5 Mutation
CCR5-Δ32 is a 32-base pair deletion mutation of the CCR5 gene in the region that corresponds to the second extracellular loop of the CCR5 receptor. The mutation renders the CCR5 receptor non-functional, which results in the reduced ability of the HIV-1 virus to enter the cell.
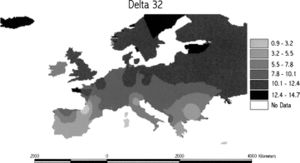
CCR5-Δ32 is common amongst Caucasian populations, typically around 10%, and is found at lower frequencies in India and the Middle East. Haplotype analysis has been used to indicate that the mutation originated approximately 700 years ago (with a range of 275-1875 years) in a single individual in northeastern Europe. A gradient cline in the allele frequencies of the mutation has been found from northern to southern Europe, suggesting that the mutation increased the survival of Caucasian populations against a strong selective factor such as a pathogen.[6] It has been hypothesized that the mutation could have conferred resistance against the Black Death and thus become favored by natural selection. However, further research has shown that the gene mutation does not provide protection against Yersinia pestis infections. [7] The current hypothesizes suggest that the mutation may have been naturally favored because it could have conferred protection against smallpox or an hemorrhagic diseases similar to the Ebola-like virus.[7]
Much speculation and research has gone into investigating the cause of the rapid spread of the mutation across Europe and to other parts of the world. Some have suggested that the mutation has been disseminated across Europe and beyond by Scandinavian Vikings during the Viking Age.[6] Others have suggested that the distribution of allele frequency may not have been caused by gene spreading, but rather by negative selection pressures resulting in the spread of pathogens during the expansion of the Roman Empire.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
1. Oppermann, M. (2004). Chemokine receptor CCR5: Insights into structure, function, and regulation. Cellular Signalling, 16(11), 1201-1210. http://www.sciencedirect.com/science/article/pii/S0898656804000786
2. Tamamis, P., & Floudas, C. A. (2014). Elucidating a key anti-HIV-1 and cancer-associated axis: The structure of CCL5 (rantes) in complex with CCR5. Sci.Rep., 4. http://www.nature.com/srep/2014/140626/srep05447/full/srep05447.html
3. Sullivan, N. L., Eickhoff, C. S., Zhang, X., Giddings, O. K., Lane, T. E., & Hoft, D. F. (2011). Importance of the CCR5–CCL5 axis for mucosal trypanosoma cruzi protection and B cell activation. The Journal of Immunology, 187(3), 1358-1368. http://www.jimmunol.org/content/187/3/1358.long
4. Tyner, J. W., Uchida, O., Kajiwara, N., Kim, E. Y., Patel, A. C., O'Sullivan, M.,P., et al. (2005). CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nature Medicine, 11(11), 1180-1187.http://www.nature.com/nm/journal/v11/n11/full/nm1303.html
5. Tamamis, P., & Floudas, C. A. (2014). Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop. Plos One, 9(4), e95767. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3999033/
6. Lucotte, G., & Dieterlen, F. (2003). More about the viking hypothesis of origin of the Δ32 mutation in the CCR5 gene conferring resistance to HIV-1 infection. Infection, Genetics and Evolution, 3(4), 293-295. http://www.sciencedirect.com/science/article/pii/S1567134803000960
7. Faure, E., & Royer-Carenzi, M. (2008). Is the european spatial distribution of the HIV-1-resistant CCR5-Δ32 allele formed by a breakdown of the pathocenosis due to the historical roman expansion? Infection, Genetics and Evolution, 8(6), 864-874. http://www.sciencedirect.com/science/article/pii/S1567134808001524
8.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
