Clostridium perfringens toxins
Overview
By [Victor Progar]
Clostridium perfringens (C. perfringens) is a common bacteria that is responsible for food poisoning as well as gas gangrene and related necrotic conditions in humans and other mammals [10]. Some other members of the genus Clostridium, which are closely related to C. perfringens, include: C botulinum, which produces the botulinum toxin responsible for botulism, C difficile, which can be a reason for diarrhea brought on by antibiotics, and C tetani, which is the causative agent behind tetanus [11]. While these species share many similarities beyond their pathogenic nature, the diseases caused by these bacteria, as well as the toxins responsible for various symptoms are a defining feature of the genus, and particularly so for C. perfringens. Among C. perfringens, there are several strains, each with unique toxin production capabilities and in turn, unique symptom displays in infected organisms. As such, C. perfringens is implicated in a wide variety of conditions, and while the role of C. perfringens in some diseases has been well established, there is currently much work being done to help elucidate the role of C. perfringens and its toxins in other diseases.
Description
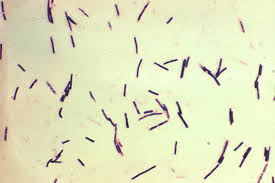
C. perfringens are rod-shaped, non-motile, anaerobic, Gram-positive, and spore-forming bacteria which are common in many different microbiota, and are found in the soil, marine sediment, in decaying vegetation, and in the intestinal tract of humans and other organisms [8]. They are thought to be among the most common pathogens in existence, and also are thought to have the shortest generation time [10]. C. perfringens is classified by strain types, separated into 5 groups, denoted A-E. These groups are established based on which particular toxins each one can produce. The major toxins used for strain classification are alpha toxin, beta toxin, epsilon toxin, and iota toxin [12]. While these four toxins are used for grouping purposes and are responsible for most of the symptoms in the variety of diseases caused by C. perfringens, strains are able to produce an assortment of other minor toxins as well as enterotoxins, which target intestinal tissue [9]. C. perfringens can cause a variety of symptoms depending on the strain and toxin present, resulting in a range of conditions from mild enteric disease to severe flesh eating necrosis and gas gangrene, which can be identified by a build up of gas produced by the bacteria in the dying tissue of an organism [3]. C. perfringens can also be present in the gut of humans and animals alike without the host individual displaying any negative signs or symptoms [10].
Genome and Metabolism
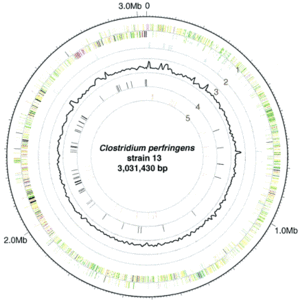
The complete genome of C. perfringens consists of a 3,031,430 bp sequence arranged in a circular chromosome [1]. Of note is the very low G+C content of the genome (28.6%) which is markedly low even for the Firmicute phylum which C. perfringens is a member of [1]. There are 2,660 protein coding regions and 10 rRNA regions [1]. In terms of metabolism, C. perfringens has a set of enzymes for glycolysis as well as enzymes for breaking down sugars including fructose, lactose, sucrose, and other compounds [1]. Genes for TCA cycle or respiratory chain enzymes or proteins were not found in the genome, as predicted based on the anaerobic nature of the bacteria [1]. Genes coding for fermentation pathway proteins and enzymes were present. With respect to amino acid biosynthesis, the genome of C. perfringens lacked genes for constructing many of the amino acids, suggesting that C. perfringens is unable to grow in nutrient poor environments [1]. A significant portion of the genome of C. perfringens is dedicated to virulence-related genes, with genes coding for several different toxins including enterotoxin, alpha, and beta toxins. Genes for virulence regulation are also present in the genome, notably the VirR/VirS regulon [1]. It should be noted that the strain sequenced for the genomic information presented was isolated from soil and identified as a type A strain [1]. Based on the information from analyzing the genetic sequence of C. perfringens, it is clear that C. perfringens needs to rapidly produce toxins and enzymes to break down host cell tissue to survive, as it lacks most of the machinery necessary to biosynthesize vital amino acids.
Strain types
There are 5 strain types of C. perfringens, which are denoted by an A-E classification. The classification is dependent upon the type of major lethal toxin the strain is able to produce. Type A bacteria produce alpha toxin, type B bacteria produce alpha, beta, and epsilon toxins, type C bacteria produce alpha and beta toxins, type D bacteria produce alpha and epsilon toxins, and type E bacteria proteins produce alpha and iota toxins [12]. Each strain is also capable of producing secondary toxins, which include, but are not limited to enterotoxin and beta 2 toxin [3].
Alpha toxin
The alpha toxin, found in type A strains of C. perfringens causes gas gangrene and also hemolysis in infected individuals [5]. The alpha toxin shares structural similarities with toxins produced by other bacteria, in addition to naturally occurring enzymes [7]. Furthermore, the alpha toxin of C. perfringens requires zinc for activation, after which the toxin binds to the surface of the host cell, whereby a series of pathways result in increased permeability in blood vessels [7]. This toxin is most commonly found in animals, and is found more frequently in humans than the other toxins [3].
Beta toxin
This lethal toxin is found in C. perfringens type B and type C strains [12]. This toxin also results in necrosis by way of increased blood pressure which is brought on by the presence of catecholiamine [2]. Beta toxin is the main disease causing agent in type B strains of C. perfringens, which usually manifests itself as enterotoxemia in infantile mammals, particularly piglets, calves, foal, and lambs [11]. This toxin is vulnerable to being degraded by proteolytic enzymes, one such enzyme being trypsin [2]. As such, in conditions where trypsin is not present, the toxin thrives [2].
Epsilon toxin
Produced by type B and type D strains of C. perfringens, epsilon toxin is most commonly isolated from animals, rarely humans [12]. Similar to the other toxins, epsilon toxin creates pores in tissues, which can result in leaked potassium ions and fluid leakage, which leads to greater complications, giving way to the symptoms associated with C. perfringens infection [12].
Iota toxin
The iota toxin is produced solely by type E strain of C. perfringens and is comprised of 2 unlinked proteins, and is known as an AB toxin because of the 2 domains, aptly named A and B, respectively [4]. In these type of toxins, one of the domains, A is usually the active portion, while the other domain, B, is the part of the toxin that binds to a receptor site on the membrane of the host cell [4]. Although the iota toxin can cause similar tissue death in infected individuals, infection from type E strains aren’t as prevalent as infections from other strains [12].
Infection
Since C. perfringens is naturally found in soil as well as other natural environments, it is unsurprising that C. perfringens is so prevalent in terms of infection among humans and animals alike. In humans, there are essentially two types of infection: food poisoning and open wound infection. While the symptoms of infection in humans are more dichotomous, being either rather mild or quite severe, in animals more severe cases of intestinal necrosis are often seen.
Food poisoning
C. perfringens is among the most common causative agents of food borne illness in humans in the United States of America, and is responsible for an estimated total of nearly 1 million cases of sickness each year [10]. The symptoms of food illness from C. perfringens include diarrhea and abdominal pain, and these symptoms arise anywhere from 6 hours to 24 hours after initial exposure [10]. Usually, the symptoms are mild and will only last for about 24 hours there about [10]. The enterotoxin in C. perfringens is the culprit for food illness. While C. perfringens can be found in contaminated animals, properly processing and cooking food can reduce the likelihood of food illness in humans. However, if the food is not properly heated, the bacteria may remain in the food to infect the unlucky diner. Interestingly enough, most cases of food illness brought on by C. perfringens go unnoticed and are frequently asymptomatic. This reason for this is that antibodies to the toxin are very common across the human population, supporting the notion that C. perfringens is very abundant and frequently infects humans and animals as well[11]. In some rare cases, strains of C. perfringens that are in spore form within an animal can germinate after a long enough period of time and can infect individuals upon consumption of the contaminated animal [11]. This can result in slightly worse cases of illness, and in some rare cases when the major toxins are produced, can result in severe necrosis of the intestine. Pig bel is one such case of serious illness which is brought on by ingestion of type C bacteria which produces beta toxin [11]. This results in ulceration and perforation of the intestine and can be fatal. This case is, thankfully, quite rare.
Traumatic open wound infection
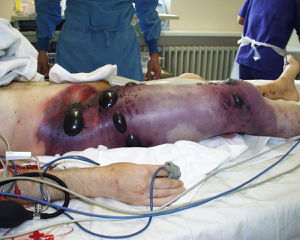
In addition to being infectious when contaminated animals are eaten without being properly processed or cooked, some of the worst symptoms as a result of C. perfringens infection are due to open wounds being contaminated. After a traumatic wound, such as one that might be suffered during a war, if soil or some other bacteria containing medium enters the wound. While this was most common in humans during wars before more recent technological innovations in the medical field, cases do still occur, though not as much in developed countries. When wounds were infected, strains of C. perfringens, particularly the alpha toxin producing type A strain, would manifest itself as gas gangrene in the affected tissues [7]. Once inside the infected individual, the bacteria produce the exotoxins (in most cases, as mentioned, alpha toxin produced from type A strains) and the toxins readily break down tissue and also help C. perfringens to outcompete other naturally occurring bacteria [7]. While these toxins break down tissues, gas is also released. Since the internal environment is favorable for rapid growth of C. perfringens, the disease can spread very quickly, often leading to septic shock and in some cases, death [10]. More vile cases of necrosis, particularly in intestines, are more common in animals rather that humans based on their close contact with soil containing C. perfringens, especially strain types B and D which produce the alpha, beta, and epsilon toxins [3].
Prevention and Treatment
When considering food borne illness brought on by consumption of animal products contaminated with C. perfringens, the best remedy for illness seems to be the proactive route of preventing infection in the first place. The best way to go about doing so is properly heating food to a reasonable temperature before ingesting depending on the animal type, and also by keeping hot foods warm (generally above 60 ºC) and refrigerating left overs at appropriately cold temperatures (below 5 ºC) [10]. However, if an individual is infected, most symptoms will generally be very mild and not require treatment. In particularly bad cases, infected individuals can be given fluids intravenously and electrolytes can be supplemented, both of which with the intention of preventing dehydration from diarrhea or vomiting when applicable [10]. C. perfringens food poisoning can be confirmed by collecting samples from an individual’s stool and by streaking and culturing the bacteria on blood agar plates and Gram staining [1].
As far as necrosis of intestines or other infected tissue (be it in animals or humans) is concerned, prevention can best be done by cleaning out open wounds, and staying clear from contaminated food, soil, or water. Once infected, however, treatment usually involves administration of antibiotics [12]. Hyperbaric oxygen therapy can also be used to help to reduce the growth rate of the bacteria, which are anaerobic [12]. Dead tissue can also be removed. In instances where necrosis is too great in tissues, amputation is sometimes the only viable option.
Current breakthroughs and future pathways for exploration
Implications in case of multiple sclerosis
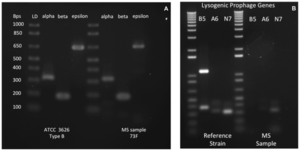
A recent study has isolated type B C. perfringens, one of the strains that produce the epsilon toxin from a patient with multiple sclerosis [6]. This was the first time that type B strain of C. perfringens was isolated from a human [6]. Given that the origins of multiple sclerosis are still unknown, the cause of the characteristic lesions and demyelination seen in those with multiple sclerosis are unclear as well [6]. Quite interestingly, the mechanism of action and effects of the epsilon toxin result in similar tissue damage seen in cases of multiple sclerosis, and findings from recent studies showed that the epsilon toxin associated with the B type C. perfringens may possibly be a trigger for multiple sclerosis [6].
Cancer treatment possibilities
Surprisingly, there are actually some potential positive implications for some of the toxins associated with C. perfringens, particularly enterotoxin, which is not one of the main lethal toxins used for classification of the different strain types. The C. perfringens enterotoxin binds to claudin receptors, which are present in great numbers on cancer cells [9]. Given that the toxin can kill cells with these receptors, researchers found that there is the potential for the enterotoxin to be used to eradicate cancerous cells with greater than normal levels of claudin receptors [9]. There is still much work that needs to be done but it is very interesting to find that there is the possibility of some good that can come from the utilization of a toxin from a notorious bacteria, C. perfringens.
References
[1 Hunter SE, Brown JE, Oyston PC, Sakurai J, Titball RW (September 1993). "Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus". Infect. Immun. 61 (9): 3958–65.
2 Jianming Chen and Bruce A. McClane Role of the Agr-Like Quorum-Sensing System in Regulating Toxin Production by Clostridium perfringensType B Strains CN1793 and CN1795. Infect Immun. Sep 2012; 80(9): 3008–3017.doi: 10.1128/IAI.00438-12PMCID: PMC3418738
3 Morris, Winston E.1 ; Dunleavy, Mariana V.1; Diodati, Julián1; Berra, Guillermo1; Fernandez-Miyakawa, Mariano E.1Effects of Clostridium perfringens alpha and epsilon toxins in the bovine gut. Anaerobe, Volume 18, issue 1 (February, 2012), p. 143-147ISSN: 1075-9964, DOI: 10.1016/j.anaerobe.2011.12.003
4 Jean-Christophe Marvaud, Bradley G. Stiles, Alexandre Chenal, Daniel Gillet, Maryse Gibert, Leonard A. Smith, and Michel R. Popoff. Clostridium perfringens Iota Toxin: MAPPING OF THE Ia DOMAIN INVOLVED IN DOCKING WITH Ib AND CELLULAR INTERNALIZATION. November 15, 2002 The Journal of Biological Chemistry, 277, 43659-43666.
5 Nowell, Victoria J., Kropinski, Andrew M., Songer, J. Glenn, MacInnes, Janet I., Parreira, Valeria R. Genome Sequencing and Analysis of a Type A Clostridium perfringens Isolate from a Case of Bovine Clostridial Abomasitis. Published: March 08, 2012. DOI: 10.1371/journal.pone.0032271
6 Rumah KR, Linden J, Fischetti VA, Vartanian T (2013) Isolation of Clostridium perfringens Type B in an Individual at First Clinical Presentation of Multiple Sclerosis Provides Clues for Environmental Triggers of the Disease. PLoS ONE 8(10): e76359. doi:10.1371/journal.pone.0076359
7 Sakurai J, Nagahama M, Oda M. (2004). "Clostridium perfringens Alpha-Toxin: Characterization and Mode of Action". J Biochem 136 (5): 569–574.
8 Tohru Shimizu, Kaori Ohtani, Hideki Hirakawa, Kenshiro Ohshima, Atsushi Yamashita, Tadayoshi Shiba, Naotake Ogasawara, Masahira Hattori, Satoru Kuhara, and Hideo Hayashi. “Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater” Proc Natl Acad Sci U S A. 2002 January 22; 99(2): 996-1001. 9 Zhijian Gao and Bruce A. McClane, “Use of Clostridium perfringens Enterotoxin and the Enterotoxin Receptor-Binding Domain (C-CPE) for Cancer Treatment: Opportunities and Challenges,” Journal of Toxicology, vol. 2012, Article ID 981626, 9 pages, 2012. doi:10.1155/2012/981626 10 http://www.cdc.gov/foodsafety/clostridium-perfingens.html 11 http://www.clostridia.net/Cperfringens.htm
12 http://www.cfsph.iastate.edu/Factsheets/pdfs/epsilon_toxin_clostridium.pdf]
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2014, Kenyon College.