Comamonas badia
Classification
Higher Order Taxa
Bacteria (Kingdom), Proteobacteria (Phylum), Betaproteobacteria (Class), Burkholderiales (Order), Comamonadaceae (Family), Comamonas(Genus)
Species
Comamonas badia
Description
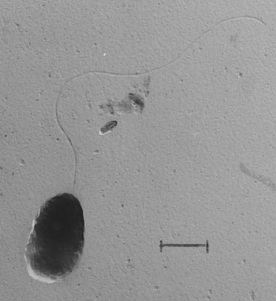
Comamonas Badia is a gram negative, rod-shaped, strictly aerobic, and highly motile bacteria with a polar flagellum and was isolated from phenol-adapted activated sludge from a factory sludge from Japan [1]. It was originally classified under the genus Pseudomonas [3][4]. Based on 16S rRNA gene sequence and G+C content, C. badia was determined to represent its own genus, Comamonas. This novel one was first represented by the species C. badia, type-strain IAM 14839T [1][9].
Comamonas badi forms pink colonies when cultured in Luria slant media and forms visible flocs in Luria broth media, unlike other Comamonas species which do not form flocs [2]. Comamonas badia grows at a very slow rate in a flocculated state with a generation time of about 12 hours at optimal temperatures (27-30°C) in LB media and produces few free-floating cells. When cultured in broths, the supernatant is usually clear [4]. This bacterium grows the slowest at 20°C (19 hours for generation time) and does not grow at 37°C (average human body temperature), unlike other Comamonas species which do undergo growth at 37°C [1]. For floc formation, C. badia grows embedded in a film mesh structure made out of mucopolysaccharides which consists of glucosamine, glucose, mannose, galactose and rhamnose. Only about 10% of the total polysaccharide content found with C. badia is involved with floc formation [3]. The ability to form flocs gives this species a protection advantage from predators compared to free roaming cells, allows other bacteria to form on the flocs, and prevents the organism from being washed out of the system that it is in, i.e. activated sludge [10].
Microbial metabolism of groundwater pollutants
Significance

It has been found that C. badia has moderate phenol tolerance and moderate phenol degradation capabilities. C. badia existing in a floc-formation is also the most predominating bacteria species in activated sludge and is involved indegrading highly toxic and carcinogenic phenol compounds in coke wastewater.[2] Being able to tolerate and degrade such toxic compounds, the ability to form flocs is an integral part this microbe in activated sludge to treat wastewaters. [10] C. badia has a unique ability in which it is responsible for its own deflocculation by producing an exoenzyme. Various commercial enzymes are unable to induce deflocculation in C. badia and therefore the bacteria’s life activities are determined by their own accord via polysaccharide-synthesizing enzymes and deflocculating enzymes.[5]
Dehalogenation
The bacterium Pseudomonas stutzeri strain KC can dehalogenate CT into carbon dioxide and chlorine without producing the toxic intermediate chloroform (CCl3H). This bacterium is originally isolated from an aquifer in Seal Beach in California. It is dependent on anaerobic conditions and in iron-limited media this bacterium produces and secretes a chelator called pyridine-2,6 (bis)thiocarboxylate (PDTC cf. figure 2.) [5]. When PDTC is in contact with a broad range of cell components it turns into a reduced form (the iron in the complex is reduced) and this is essential for its extracellular activity. PDCT has to be in a complex with copper in order for the fast turnover rate of CT into CO2. This complex functions both as a reactant and a catalyst in the reaction. When Pseudomonas stutzeri is in environments were nitrate is present as the electron acceptor a more rapid production of PDTC is observed [6].
Denitrification
In many agricultural areas in North America the nitrate concentrations exceed the standards. Denitrifying organisms are capable of using nitrate or nitrite as terminal electron acceptors thereby removing the excess of nitrogen from the environment. The organism Methylomirabilis oxyfera is an example of such an organism. This denitrifying bacterium is special in that it doesn’t have the gene encoding nitrous oxide reductase, the protein that converts N2O to N2. Instead they harbor an operon which encodes the complete methane monooxygenase complex. This enables it to oxide methane in an aerobic pathway [1]. The mechanism takes advances of the oxidation of methane to drive denitrification. They do so by producing oxygen from nitrite via nitrite oxide (thereby bypassing the intermediate nitrous oxide) and then use this oxygen to oxide methane in an anaerobic environment. This is called nitrite dependent anaerobic methane oxidation. The overall redox reaction is 3CH4 + 8NO2- + 8H+ -> 3CO2 + 4N2 + 10 H2O. In this way the organism uses the potent greenhouse gas methane and reduces nitrite thereby contributing to the removal of excess N-compounds in groundwater [1].
Treatment technologies
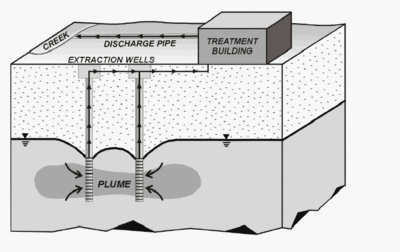
Contamination of groundwater can lead to severe health problems and environmental changes if left untreated. Bioaugmentation is a widespread biological technique used in the removal of chlorinated compounds. By introducing natural electron donors that are helpful in the removal of halogenated compounds into the groundwater the growth of dehalorespiring organisms can be favored. Optional conditions for dehalogenation are provided without any engineering steps taken [7]. Pump and treat method is also one of the most used groundwater remediation techniques. Removal of contaminated groundwater from soil with the use of pumps followed by subsequent remediation at the surface helps overcome the persistence of the pollutants (cf. figure 3). It is typically biological or chemical treatments that remove the pollutants. This method is costly and slow however and some contaminants cannot be removed because they stick to soil and rocks or are not sufficient water soluble [4].
References
(1) Tago Y., Yokota A. “Comamonas badia sp nov., a Floc-forming Bacterium Isolated from Activated Sludge.” Journal of General and Applied Microbiology, 2004, DOI: 10.2323/jgam.50.243
(2) Felföldi T., Székely A., Gorál R., Barkács K., Scheirich G., András J, Rácz A., Márialigeti K. “Polyphasic Bacterial Community Analysis of an Aerobic Activated Sludge Removing Phenols and Thiocyanate from Coke Plant Effluent.” Bioresource Technology, 2010, DOI: 10.1016/j.biortech.2009.12.053
(3) Tago Y., Aida K. “Exocellular Mucopolysaccharide Closely Related to Bacterial Floc Formation” Applied and Environmental Microbiology, 1977, ISSN: 0099-2240
(4) Tago Y., Kuraishi H., Aida K. “The Formation of a Model Floc able to Decompose Phenol by the Mixed Culture of Bacteria Isolated from Activated Sludge.” Journal of General and Applied Microbiology, 1975, DOI: 10.2323/jgam.21.41
(5) Tago Y., Aida K. “The Deflocculating Enzyme Produced by a Floc-Forming Bacterium.” Journal of General and Applied Microbiology, 1975, DOI: 10.2323/jgam.21.365
(6) Blöthe M., Roden E. “Composition and Activity of an Autotrophic Fe(II)-Oxidizing, Nitrate-Reducing Enrichment Culture” Applied and Environmental Microbiology, 2009, DOI: 10.1128/AEM.01742-09
(7) Mahendran B., Lishman L., Liss S. “Structural, Physicochemical and Microbial Properties of Flocs and Biofilms in Integrated Fixed-Film Activated Sludge (IFFAS) Systems” Water Research (Oxford), 2012, DOI: 10.1016/j.watres.2012.05.058
(8) Zita A., Hermansson M. “Effects of Bacterial Cell Surface Structures and Hydrophobicity on Attachment of Activated Sludge Flocs” Applied and Environmental Microbiology, 1997, ISSN: 0099-2240
(9) Stackebrandt E., Goebel B. “Taxonomic Note: A place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology” International Journal of Systematic Bacteriology, 1994, ISSN: 0020-7713
(10) Raszka A., Chorvatova M., Wanner J. “The Role and Significance of Extracellular Polymers in Activated Sludge. Part I: Literature Review” Acta Hydrochimica et Hydrobiologica (Weinheim an der Bergstrasse, Germany), 2006, DOI: 10.1002/aheh.200500640