Deinococcus radiodurans: Extreme Radiation Tolerance: Difference between revisions
No edit summary |
No edit summary |
||
| Line 31: | Line 31: | ||
Radiation is caused by electromagnetic waves and the results on a cell are oxidation damage to proteins, DNA, RNA, and enzymes [[#References|[2]]]. One of the main causes of cell death is due to the double stranded breaks (DSBs) that occur in the DNA [[#References|[4]]]. Unlike most other bacteria, ''D. radiodurans'' retains its viability in radiation. It can withstand up to 21,000 J/m2 of ultraviolet radiation which usually results in cyclobutane dimers and pyrimidine-pyrimidone photoproducts which cause CC-TT or C-T base pairing Schematic of origin of different types of radiation and its effects on extremophiles. These bacteria can also withstand 5 kGy of Gamma radiation without loss of viability [[#References|[4]]]. | Radiation is caused by electromagnetic waves and the results on a cell are oxidation damage to proteins, DNA, RNA, and enzymes [[#References|[2]]]. One of the main causes of cell death is due to the double stranded breaks (DSBs) that occur in the DNA [[#References|[4]]]. Unlike most other bacteria, ''D. radiodurans'' retains its viability in radiation. It can withstand up to 21,000 J/m2 of ultraviolet radiation which usually results in cyclobutane dimers and pyrimidine-pyrimidone photoproducts which cause CC-TT or C-T base pairing Schematic of origin of different types of radiation and its effects on extremophiles. These bacteria can also withstand 5 kGy of Gamma radiation without loss of viability [[#References|[4]]]. | ||
[[File:wiki | [[File:wiki fig 2.jpg|300px|thumb|right| Figure 2. Schematic of origin of different types of radiation and its effects on extremophiles [[#References|[2]]]]] | ||
''D. radiodurans'' does not have a mechanism to prevent DSBs, it only has mechanisms to repair the DNA after these breaks have occurred [[#References|[4]]]. The genome is in a condensed ring to prevent diffusion of the DSBs [[#References|[4]]]. Following exposure to radiation, cellular regulatory mechanisms prevent replication of DNA [[#References|[4]]]. Proteins called ddrA and pprA then bind to the ends of all the DNA breaks to protect them from degradation by nucleases [5]. The DSB ends are then processed by UvrD and Rec J into 3’ single strands of DNA [[#References|[7]]]. RecFOR loads RecA onto DSBs which searches for double stranded DNA homology and also primes DNA repair synthesis on partially overlapping fragments of the homologous DSB which are to be used as templates [[#References|[7]]]. The 3’ ends of a DSB goes in between strands of a matching unbroken strand and each end is base paired by Pol A and then joins the other annealing strand [[#References|[4]]] (see Figure 3). | ''D. radiodurans'' does not have a mechanism to prevent DSBs, it only has mechanisms to repair the DNA after these breaks have occurred [[#References|[4]]]. The genome is in a condensed ring to prevent diffusion of the DSBs [[#References|[4]]]. Following exposure to radiation, cellular regulatory mechanisms prevent replication of DNA [[#References|[4]]]. Proteins called ddrA and pprA then bind to the ends of all the DNA breaks to protect them from degradation by nucleases [5]. The DSB ends are then processed by UvrD and Rec J into 3’ single strands of DNA [[#References|[7]]]. RecFOR loads RecA onto DSBs which searches for double stranded DNA homology and also primes DNA repair synthesis on partially overlapping fragments of the homologous DSB which are to be used as templates [[#References|[7]]]. The 3’ ends of a DSB goes in between strands of a matching unbroken strand and each end is base paired by Pol A and then joins the other annealing strand [[#References|[4]]] (see Figure 3). | ||
Revision as of 01:05, 25 November 2013
Classification
Higher Order Taxa
Bacteria (Domain); Deinococcus-Thermus (Phylum); Deinococcus (Class); Deinococcales (Order); Deinococcaceae (Family); Deinococcus (Genus)
Species
radiodurans
Description
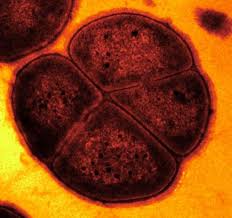
Deinococcus radiodurans was originally discovered in 1956 by Anderson et al. who noticed the presence of bacteria in a can of ground beef that was sterilized by gamma irradiation [8]. Deinococcus radiodurans was first classified as Micrococcus radiodurans, due to its similarities to that genus. However, after further research, it was later put into a phylum of its own [4]. “Deinos”, in Greek, means strange or unusual, which is a good description of this bacteria [7]. It is a spherical, non-motile, non-spore forming bacterium with a red pigment [6]. This gram positive bacteria is 1.5-3.5 μm in diameter and often forms tetrads but can also be found in singles of pairs [6]. Its cellular envelope more closely resembles that of a gram negative bacterium as it has 6 layers- (from inside out) cell membrane, peptidoglycan, compartmentalized layer, electron transport zone, outer membrane and a hexagonally packed intermediate layer [3]. The genome of D. radiodurans was sequenced and published in 1999 by TIGR. D. radiodurans is novel due to its ability to survive in extremely high levels of radiation [8].
Ecology and Significance
The natural environment of Deinococcus radiodurans is not well defined [1]. It has been isolated around the world in places with high concentrations of organic nutrients, such as in animal feces, soil, sewage, and processed meats [6]. They also have been isolated from Fukushima Daiichi and Chernobyl both locations of nuclear accidents [2]. There are four species that make up the Deinococcus genus: Deinococcus radiodurans, Deinococcus proteolyticus, Deinococcus radiopugnans, and Deinococcus radiophilus [6]. The Deinoccoceae lineage all have a degree of radiation resistance but also may individually be a mesophile, thermophile, psychrophile, or a combination of more than one, surviving in environments from hot springs, to deserts, to Antarctica [7]. Out of these, D. radiodurans is the most extreme in terms of radiation resistance and desiccation tolerance [1]. Cyanobacteria such as Chroococcidiopsis and fungi such as Filobasidiales also have the characteristic of radiation resistance but not to the extent of D. radiodurans [7]. D10, the dose required to kill 90% of population is 0.005 kGy for humans, 0.25 kGy for Escherichia coli and can be as high as around 10 kGy for D. radiodurans [1]. There are no natural environments that have radiation greater than 400 mGy per year so their resistance is more likely due to the fact that they have genes that allow them to survive desiccation, which causes double-stranded breaks [4].
Genome Structure and Mechanisms of Resistance
Radiation is caused by electromagnetic waves and the results on a cell are oxidation damage to proteins, DNA, RNA, and enzymes [2]. One of the main causes of cell death is due to the double stranded breaks (DSBs) that occur in the DNA [4]. Unlike most other bacteria, D. radiodurans retains its viability in radiation. It can withstand up to 21,000 J/m2 of ultraviolet radiation which usually results in cyclobutane dimers and pyrimidine-pyrimidone photoproducts which cause CC-TT or C-T base pairing Schematic of origin of different types of radiation and its effects on extremophiles. These bacteria can also withstand 5 kGy of Gamma radiation without loss of viability [4].
]
D. radiodurans does not have a mechanism to prevent DSBs, it only has mechanisms to repair the DNA after these breaks have occurred [4]. The genome is in a condensed ring to prevent diffusion of the DSBs [4]. Following exposure to radiation, cellular regulatory mechanisms prevent replication of DNA [4]. Proteins called ddrA and pprA then bind to the ends of all the DNA breaks to protect them from degradation by nucleases [5]. The DSB ends are then processed by UvrD and Rec J into 3’ single strands of DNA [7]. RecFOR loads RecA onto DSBs which searches for double stranded DNA homology and also primes DNA repair synthesis on partially overlapping fragments of the homologous DSB which are to be used as templates [7]. The 3’ ends of a DSB goes in between strands of a matching unbroken strand and each end is base paired by Pol A and then joins the other annealing strand [4] (see Figure 3).
Recombinational Repair is possible due to the fact that this bacterium has a segmented genome and has 4-10 copies of the genome in each cell [4]. Along with making it very unlikely that every copy will be severely damaged, it can use the multiple copies to homologously recombine, repairing its genome [5]. RecA is the protein used for dependant homologous recombination [10]
Mn (II) is also required for the cells survival as it facilitates the cleanup of Reactive Oxygen Species (ROS) which are produced during radiation [7]. These ROS are what damage lipids, proteins, nucleic acids, carbohydrates and create DSBs [7].
Metabolism
Ignicoccus species are chemolithoautotrophs that use molecular hydrogen as the inorganic electron donor and elemental sulphur as the inorganic terminal electron acceptor[1] . The reduction of the elemental sulphur results in the production of hydrogen sulphide gas.
Ignicoccus are autotrophs in that they fix their own carbon dioxide into organic molecules. The carbon dioxide fixation process they use is a novel process called a dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle that involves 14 different enzymes[8] .
Members of the Ignicoccus genus are able to use ammonium as a nitrogen source.
Growth Conditions
Because members of the Ignicoccus genus are hyperthermophiles and obligate anaerobes, it is not surprising that their growth conditions are very complex. They are grown in a liquid medium known as ½ SME Ignicoccus which is a solution of synthetic sea water which is then made anaerobic.
Grown in this media at their optimal growth temperature of 90C, the members of the Ignicoccus genus typically reach a cell density of ~4x107cells/mL[1] .
The addition of yeast extract to the ½ SME media has been shown to stimulate the growth and increase maximum cell density achieved. The mechanism by which this is achieved is not known[1] .
Symbiosis
Ignicoccus hospitalis is the only member of the genus Ignicoccus that has been shown to have an extensive symbiotic relationship with another organism.
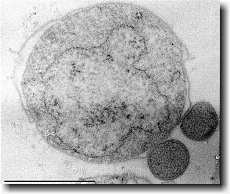
Ignicoccus hospitalis has been shown to engage in symbiosis with Nanoarchaeum equitans . Nanoarchaeum equitans is a very small coccoid species with a cell diameter of 0.4 µm[9] . Genome analysis has provided much of the known information about this species.
To further complicate the symbiotic relationship between both species, it’s been observed that the presence of Nanoarchaeum equitans on the surface of Ignicoccus hospitalis somehow inhibits the cell replication of Ignicoccus hospitalis . How or why this occurs has not yet been elucidated[3] .
Nanoarchaeum equitans
Nanoarchaeum equitans has the smallest non-viral genome ever sequenced at 491kb[9] . Analysis of the genome sequence indicates that 95% of the predicted proteins and stable RNA molecules are somehow involved in repair and replication of the cell and its genome[3] .
Analysis of the genome also showed that Nanoarchaeum equitans lacks nearly all genes known to be required in amino acid, nucleotide, cofactor and lipid metabolism. This is partially supported by the evidence that Nanoarchaeum equitans has been shown to derive its cell membrane from its host Ignicoccus hospitalis cell membrane. The direct contact observed between Nanoarchaeum equitans and Ignicoccus hospitalis is hypothesized to form a pore between the two organisms in order to exchange metabolites or substrates (likely from Ignicoccus hospitalis towards Nanoarchaeum equitans due to the parasitic relationship). The exchange of periplasmic vesicles is not thought to be involved in metabolite or substrate exchange despite the presence of vesicles in the periplasm of Ignicoccus hospitalis .
These analyses of the Nanoarchaeum equitans genome support the fact of the extensive symbiotic relationship between Nanoarchaeum equitans and Ignicoccus hospitalis. However, it has not yet been proven that it is a strictly parasitic relationship and further research may prove that there is a commensal relationship between the two species.
References
(1) Burggraf S., Huber H., Mayer T., Rachel R., Stetter K.O. and Wyschkony I. ” Ignicoccus gen. nov., a novel genus of hyperthermophilic, chemolithoautotrophic Archaea, represented by two new species, Ignicoccus islandicus sp. nov. and Ignicoccus pacificus sp. nov.” International Journal of Systematic and Evolutionary Microbiology, 2000, Volume 50.
(2) Naether D.J. and Rachel R. “The outer membrane of the hyperthermophilic archaeon Ignicoccus: dynamics, ultrastructure and composition.” Biochemical Society Transactions, 2004, Volume 32, part 2.
(3) Giannone R.J., Heimerl T., Hettich R.L., Huber H., Karpinets T., Keller M., Kueper U., Podar M. and Rachel R. “Proteomic Characterization of Cellular and Molecular Processes that Enable the Nanoarchaeum equitans- Ignicoccus hospitalis Relationship.” PLoS ONE, 2011, Volume 6, Issue 8.
(4) Eisenreich W., Gallenberger M., Huber H., Jahn U., Junglas B., Paper W., Rachel R. and Stetter K.O. “Nanoarchaeum equitans and Ignicoccus hospitalis: New Insights into a Unique, Intimate Association of Two Archaea.” Journal of Bacteriology, 2008, DOI: 10.1128/JB.01731-07.
(5) Grosjean E., Huber H., Jahn U., Sturt H, and Summons R. “Composition of the lipids of Nanoarchaeum equitans and their origin from its host Ignicoccus sp. strain KIN4/I.” Arch Microbiol, 2004, DOI: 10.1007/s00203-004-0725-x.
(6) Briegel A., Burghardt T., Huber H., Junglas B., Rachel R., Walther P. and Wirth R. “Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell–cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography.” Arch Microbiol, 2008, DOI 10.1007/s00203-008-0402-6.
(7) Burghardt T., Huber H., Junglas B., Naether D.J. and Rachel R. “The dominating outer membrane protein of the hyperthermophilic Archaeum Ignicoccus hospitalis: a novel pore-forming complex.” Molecular Microbiology, 2007, Volume 63.
(8) Berg I.A., Eisenreich W., Eylert E., Fuchs G., Gallenberger M., Huber H.,Jahn U. and Kockelkorn D. “A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis.” PNAS, 2008, Volume 105, issue 22.
(9) Brochier C., Gribaldo S., Zivanovic Y., Confalonieri F. and Forterre P. “Nanoarchaea: representatives of a novel archaeal phylum or a fast-evolving euryarchaeal lineage related to Thermococcales?” Genome Biology 2005, DOI:10.1186/gb-2005-6-5-r42.
(10) Huber H., Rachel R., Riehl S. and Wyschkony I. “The ultrastructure of Ignicoccus: Evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon.” Archaea, 2002, Volume 1.