Ebola Transmission: Difference between revisions
Vgawlik6782 (talk | contribs) |
Vgawlik6782 (talk | contribs) |
||
| Line 45: | Line 45: | ||
<br>Due to its classification as a level four safety agent, studying Ebola in the lab is no small feat. The virus is not only well adapted to infect its hosts, but also excels at spreading from host to host. Scientists who choose to work with Ebola must use the utmost caution to prevent the virus' ability to infect them while they conduct their experiments. Full hazard suits must be worn at all times and each suit is throughly examined before entering a containment space.<br> | <br>Due to its classification as a level four safety agent, studying Ebola in the lab is no small feat. The virus is not only well adapted to infect its hosts, but also excels at spreading from host to host. Scientists who choose to work with Ebola must use the utmost caution to prevent the virus' ability to infect them while they conduct their experiments. Full hazard suits must be worn at all times and each suit is throughly examined before entering a containment space.<br> | ||
The best preventative measure for Ebola came about several years ago in the form of a vaccination. The only downside to this approach is that Ebola outbreaks are random and occur in undefined locations during the span of one year. Many individuals in Africa do not receive vaccines upon birth due to the cost and are therefore not protected against the virus when it has its resurgences. In addition, the vaccination series lasts six months while the disease can often be fatal in less than half of that time.<sup>[6]</sup> These two factors taken together make coming up with a lasting and final solution to the Ebola problem a difficult task for scientists.<br> | The best preventative measure for Ebola came about several years ago in the form of a vaccination. The only downside to this approach is that Ebola outbreaks are random and occur in undefined locations during the span of one year. Many individuals in Africa do not receive vaccines upon birth due to the cost and are therefore not protected against the virus when it has its resurgences. In addition, the vaccination series lasts six months while the disease can often be fatal in less than half of that time.<sup>[http://www.nature.com/srep/2012/121115/srep00811/full/srep00811.html 6]</sup> These two factors taken together make coming up with a lasting and final solution to the Ebola problem a difficult task for scientists.<br> | ||
The most attractive preventative solution at this point in time seems to be the formation of a nonreplicating subunit vaccine. This is the ideal candidate because the vaccine is administered in one dosage and has been shown to be effective in preventing infection in up to 80% of exposed test animals. This is a huge advancement and turn around from the diseases' unusually high mortality rate. This particular treatment garners extra support due to the fact that it can be stockpiled and stay effective for long periods of time. This indicates that the vaccine can be pulled out when the first signs of the disease is seen in a population and thus save many lives.<sup>[5]</sup> | The most attractive preventative solution at this point in time seems to be the formation of a nonreplicating subunit vaccine. This is the ideal candidate because the vaccine is administered in one dosage and has been shown to be effective in preventing infection in up to 80% of exposed test animals. This is a huge advancement and turn around from the diseases' unusually high mortality rate. This particular treatment garners extra support due to the fact that it can be stockpiled and stay effective for long periods of time. This indicates that the vaccine can be pulled out when the first signs of the disease is seen in a population and thus save many lives.<sup>[http://www.pnas.org/content/early/2011/11/29/1117715108 5]</sup> | ||
<br> | <br> | ||
Revision as of 06:05, 29 April 2013
Introduction
The Ebola virus is a relatively recently described, severe disease-causing pathogen that poses a huge threat to human health mostly within central Africa. This is, in part, due to its high mortality4 and lack of affordable treatment options. Ebola is considered a Biosafety level 4 (BSL-4) agent; classifying it among the most threatening pathogens that exist in the world today. Agents within this category pose severe threats to human health and can be fatal due to the lack of available vaccines and/or treatment options. There are five known Ebola species within the family Filoviridae. Four of these are disease causing in humans and are endemic to Africa6. Additionally, all of the species within the family cause varying degrees of viral hemorrhagic fever illnesses.4
Virology
Structure
The Ebola virus has nonsegmented negative RNA strand genome, which codes for at least seven proteins, enclosed in a nucleocapsid3. Four of the virus’ proteins are thought to comprise the capsid that allows the virus to survive in environments while it is not infecting hosts. The capsid allows the transcription and the translation of the viral genome and is therefore the principle player in the virus’ pathogenicity4.
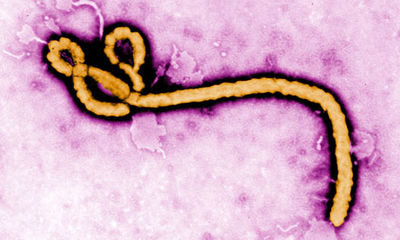
The virus has many long rods and is much longer than it is wide and is often photographed in a hooked or curved form. The virus is around 80nm in diameter and can be of varying lengths ranging from 600-1400nm1. The virus has been known to infect many different cell types including monocytes, macrophages, dendritic cells, liver cells, and endothelial cells, making it a superior infectious agent.
Infection Method
Ebola seems to be spread through direct contact with individuals, dead or alive, who possess the pathogen. In recent study it has been indicated that the Ebola virus has developed several customized ways to enter host cells depending on the virus' size and what kind of cell the virus wants to gain access to1. However, it seems in most cases the virus prefers to use macropincytosis, a form of endocytosis, to gain preliminary access. The virus requires a special cholesterol transporter known as Niemann-Pick C1 (NPC1) to successfully enter a cell and seems to be tied to the release of the genome into the host cell8. Ebola infects a wide range of cells but the most common are mononuclear phagocytes, hepatocytes, and endothelial cells. The virus has been shown to impair the immune function of the body when it infects the phagocytic cells by way of interfering with the neutrophils of the body and thus contributing its high mortality rate9. Like most pathogenic agents, the functionality of a host cell is disrupted by the presence of Ebola and eventually the virus takes over all cell function to produce only more viral copies. Once inside of a cell Ebola does not produce its own cell membrane and instead steals some of its host membrane while budding off from the host to survive and continue infection42. The virons move to new sites of infection in the host and cause major damage to cells as they go.
Pathogenesis
Immune Response
One of the proteins coded on Ebola's genome is for a glycoprotein; specifically, the viron envelope glycoprotein. It is thought to allow the virus to infect macrophages and monocytes where presence of the virus can induce the release of cytokines associated with inflammation and fever, hallmarks of the virus, and into endothelial cells, which may damage the vascular integrity of the cells. It is thought that this glycoprotein alters immune response by stoping neutrophil (a type of white blood cell) activation, while the transmembrane glycoprotein may also contribute to Ebola symptoms by targeting virus to cells of the reticuloendothelial network and the lining of blood vessels10.
Transmission
In the past it was thought that the virus only infected and caused death
in humans and non-human primates mostly in the central African region.
The disease has been known to spread from human to human by way of
contaminated bodily fluids which happens to be more common in developing nations.
Additionally, the main reservoirs of infection was
thought to be the fruit bat6. But, in recent years, as more
study is being conducted on the virus, it seems that there is more to its
complex interactions among multiple species. It was found that Ebola can, and does, infect pigs and can pass on to nonhuman primates.6 This is an early sign that pigs may play a role in human infection processes. However, in the pig the virus seems to be airborne, a dramatic change from what was previously known about the disease. This has far reaching consequences for treatment and containment.6
Infection Management
Due to its classification as a level four safety agent, studying Ebola in the lab is no small feat. The virus is not only well adapted to infect its hosts, but also excels at spreading from host to host. Scientists who choose to work with Ebola must use the utmost caution to prevent the virus' ability to infect them while they conduct their experiments. Full hazard suits must be worn at all times and each suit is throughly examined before entering a containment space.
The best preventative measure for Ebola came about several years ago in the form of a vaccination. The only downside to this approach is that Ebola outbreaks are random and occur in undefined locations during the span of one year. Many individuals in Africa do not receive vaccines upon birth due to the cost and are therefore not protected against the virus when it has its resurgences. In addition, the vaccination series lasts six months while the disease can often be fatal in less than half of that time.6 These two factors taken together make coming up with a lasting and final solution to the Ebola problem a difficult task for scientists.
The most attractive preventative solution at this point in time seems to be the formation of a nonreplicating subunit vaccine. This is the ideal candidate because the vaccine is administered in one dosage and has been shown to be effective in preventing infection in up to 80% of exposed test animals. This is a huge advancement and turn around from the diseases' unusually high mortality rate. This particular treatment garners extra support due to the fact that it can be stockpiled and stay effective for long periods of time. This indicates that the vaccine can be pulled out when the first signs of the disease is seen in a population and thus save many lives.5
Conclusion
In today's world Ebola is still a huge problem that faces many developing nations within Africa. In 2012 alone there were 5 outbreaks of Hemorrhagic Fevers around the world and 3 of them were due to the Ebola virus.
One of the factors that makes this disease so clinically challenging is the fact that it is indistinguishable from other agents that cause alternative forms of hemorrhagic fevers[3]. Because some of these diseases are treatable doctors in at risk areas must depend on detailed patient history accounts which can be a roadblock due to language barriers.
The best way to move forward from the pain caused by all of the species of Ebola is to continue research on promising single-step vaccines and begin to move them into human trial stages. The fact that some strains of Ebola can have up to an 88%[3][5] mortality rate should be enough of an incentive to push research forward. In addition, the world as a whole needs to start to get serious about ways to make affordable, life-saving vaccinations available to those who need them most.
References
3. "Ebola virus: from discovery to vaccine". Nature Reviews Immunology 3. 2003. p. 667- 85.
Edited by (Victoria Rose Gawlik), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.