Elysia chlorotica: Difference between revisions
| Line 25: | Line 25: | ||
[[File:Elysia starvation.png|thumb|300px|left|Figure 3. <b>(A)</b> Specimen of <i>V. litorea.</i> <b>(B)</b> <i>E. chlorotica</i> starved of algal food source for 2 month <b>(C)</b>] Starved for 8 month <b>(D-F)</b> .er,Endoplasmicreticulum(ER);1,lipiddeposit;p,pyrenoid;pg,plastoglobuli;t,thylakoid https://doi.org/10.1073/pnas.93.22.12333.]] | [[File:Elysia starvation.png|thumb|300px|left|Figure 3. <b>(A)</b> Specimen of <i>V. litorea.</i> <b>(B)</b> <i>E. chlorotica</i> starved of algal food source for 2 month <b>(C)</b>] Starved for 8 month <b>(D-F)</b> .er,Endoplasmicreticulum(ER);1,lipiddeposit;p,pyrenoid;pg,plastoglobuli;t,thylakoid https://doi.org/10.1073/pnas.93.22.12333.]] | ||
Kleptoplasty is an unusual behavior because the chloroplasts can remain temporarily functioning in the organism, which allows an animal to perform photosynthesis like plants. Recent research suggests that kleptoplasty can be obtained through lateral gene transfer from algae to the sea slug. The symbiotic chloroplasts in the organism remain functioning after 8 months’ starvation (even turning the organism yellowish Figure 3.C); and it is the longest-lived symbiosis of chloroplasts ever reported. Scientists suggest three possibilities to explain this phenomenon: (i) All plastid and nuclear-encoded chloroplast proteins remain stable in the organism, (ii) at the initial stage of symbiotic evolution, some algal nuclear genes may have been transferred into sea slug’s nuclear genome, or (iii) the symbiotic plastids can live individually and remain functioning without the algal cell nucleus and cytoplasm. By isolating <i>E. chlorotica</i> DNA free | Kleptoplasty is an unusual behavior because the chloroplasts can remain temporarily functioning in the organism, which allows an animal to perform photosynthesis like plants. Recent research suggests that kleptoplasty can be obtained through lateral gene transfer from algae to the sea slug. The symbiotic chloroplasts in the organism remain functioning after 8 months’ starvation (even turning the organism yellowish Figure 3.C); and it is the longest-lived symbiosis of chloroplasts ever reported. Scientists suggest three possibilities to explain this phenomenon: (i) All plastid and nuclear-encoded chloroplast proteins remain stable in the organism, (ii) at the initial stage of symbiotic evolution, some algal nuclear genes may have been transferred into sea slug’s nuclear genome, or (iii) the symbiotic plastids can live individually and remain functioning without the algal cell nucleus and cytoplasm. By isolating <i>E. chlorotica</i> DNA free from interfering mucus<ref>Rumpho,M.E.,Mujer,C.V.,Andrews,D.L.,Manhart,J.R.& | ||
Pierce, S. K. 1994. BioTechniques 17, 1097-1101.</ref>, researchers concluded the algal chloroplast gene | Pierce, S. K. 1994. BioTechniques 17, 1097-1101.</ref>, researchers concluded the algal chloroplast gene actively transcripts and translates in the sea slug. Genes such as <i>rbcL, rbcS, psaB, psbA,</i> and <i>16S rRNA</i> have been found in the organism after months of starvation<ref>Mujer, C. V., D. L. Andrews, J. R. Manhart, S. K. Pierce, and M. E. Rumpho. 1996. “Chloroplast Genes Are Expressed during Intracellular Symbiotic Association of Vaucheria Litorea Plastids with the Sea Slug Elysia Chlorotica.” Proceedings of the National Academy of Sciences 93 (22): 12333–38. https://doi.org/10.1073/pnas.93.22.12333.</ref>. <i>RbcL gene</i>, which encode Rubisco LS, has been found in E. chlorotica DNA; Rubisco LS protein is also presence after two month’s starvation. PsbA Genes were remain constant until 4 months of starvation and start to decline. The <i>16S rRNA</i> gene stays at a constant level from start to finish. <i>RbcS</i> is also remains at a steady level. These genes are directly engage in chloroplast encoding which indicates <i>E. chlorotica</i> can constantly encoding new symbiotic chloroplast using gene information even without food (chloroplast) source. Furthermore, the transcription and translation of chloroplast occurred in the situation where plant nucleocytoplasm is absent; which means the regulation of chloroplast gene expression is taken by sea slug’s nucleus and cytosol; or completely regulate within the symbiotic plastid genome. | ||
Another research about <i>E. Cholotica</i>'s encoded gene and complex provide further molecule evidence of horizontal gene transfer between multicellular eukaryotes. Three <i>V. litorea</i> nuclear-encoded genes [fucoxanthin chlorophyll <i>a/c</i>-binding protein (<i>fcp</i>) and light-harvesting complex 1 and 2 (<i>Lhcv1 and 2</i>)] had been found in sea slug's genomic DNA, primarily serve the function of support the chloroplast endosymbiosis in <i>E. chlorotica</i><ref>Schwartz, Julie A., Nicholas E. Curtis, and Sidney K. Pierce. 2010. “Using Algal Transcriptome Sequences to Identify Transferred Genes in the Sea Slug, Elysia Chlorotica.” Evolutionary Biology 37 (1): 29–37. https://doi.org/10.1007/s11692-010-9079-2.</ref>. | Another research about <i>E. Cholotica</i>'s encoded gene and complex provide further molecule evidence of horizontal gene transfer between multicellular eukaryotes. Three <i>V. litorea</i> nuclear-encoded genes [fucoxanthin chlorophyll <i>a/c</i>-binding protein (<i>fcp</i>) and light-harvesting complex 1 and 2 (<i>Lhcv1 and 2</i>)] had been found in sea slug's genomic DNA, primarily serve the function of support the chloroplast endosymbiosis in <i>E. chlorotica</i><ref>Schwartz, Julie A., Nicholas E. Curtis, and Sidney K. Pierce. 2010. “Using Algal Transcriptome Sequences to Identify Transferred Genes in the Sea Slug, Elysia Chlorotica.” Evolutionary Biology 37 (1): 29–37. https://doi.org/10.1007/s11692-010-9079-2.</ref>. | ||
Revision as of 01:34, 9 December 2021
Introduction
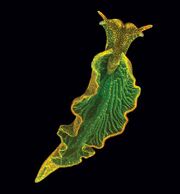
Elysia chlorotica, or the eastern emerald elysia, is a marine Gastropoda species in the Plankobranchidae family and the Sacoglossa clade. Elysia chlorotica (E. chlorotica) has been found in salt water, tidal marshes, shallow creeks, and ponds off the coast of the eastern states and in Nova Scotia, Canada at depths of 0m to 0.5m. [1] The clade that E. chlorotica belongs to are sea slugs that are able to perform photosynthesis, known as "solar-powered sea slugs"[2]. Species in this clade can perform an unusual phenomenon called "kleptoplasty", which means they can use living chloroplasts for their own energy production. After E. chlorotica first feed on algae, the chloroplast are preserved in their special digestive system and used for photosynthesis. The chloroplast remains in E. chlorotica and turns them into bright green.
Life cycle and Morphology

E. chlorotica is bilaterally symmetrical like a leaf. Their morphology and green color lets them blend into the marsh environment easily. E. chlorotica's color is largely dependent on the concentration of chlorophyII preserves in the digestive gland; Therefore, they will occasionally appear reddish and greyish in color due to lack of chloroplast[3]. The adult individual are around 3~5 centimeters long, some individuals can grow up to 6cm in length but are more commonly found between 2~3cm. An E. chlorotica has four stages of development: larval, juvenile, young adult and mature adult stages, which can be distinguish by its morphology and coloring. The average life span for E. chlorotica is 11 months, ending just after adults lay their string of eggs in the spring of each year. An annual viral expression in this species caused a synchronized death of all the adults in the population. It happens every generation both in wild and laboratory environments [4].
Larval Stage
E. chlorotica's life start with veliger larva. They have a shell and ciliated vellum used for swimming and obtaining food.[5] In the early stage, E. chlorotica larva feeds on small phytoplankton in the ocean water. Larva has two eyespots on each side of its mouth. The Ciliated vellumon at the outside of the mouth can constantly bring food into the digestive tract to the stomach. Then, food will be sorted and filtered in this process before it moves on to the digestive gland. In digestive gland, nutrients in the food will be absorb by E. chlorotica (Figure 2.A).[6]
Juvenile Stage
During juvenile stage, newly hatched E. chlorotica are usually translucent brown with red spots on their bodies. This stage remains until the initial feeding on algae, Vaucheria litorea (Figure 2.B).
Young/Mature Adult Stage
Elysid refers to the adult slug’s leaf-like shape which is caused by two large lateral parapodia (a pair of fleshy protrusions like wings for swimming) on either side of its body. This morphology is beneficial as both camouflage and allowing the slug to be more efficient at photosynthesis. Other members of this family are distinguished by their parapodia in addition to bright coloring. The E. chlorotica obtains chloroplasts from the algae to preserve in its special digestive tract. The presence of chloroplasts gradually turn the body color into bright green, making it lose its red spots. It takes 3~5 days for E. chlorotica to reach the threshold and transform to the mature adult stage (Figure 2.D). Before that, some scientists will identify that period as a short young adult or intermediate stage (Figure 2.C)[7].
Sexual Reproduction
E. chloroticas are simultaneous hermaphrodites, which means they contain both female and male reproductive organs. It is theoretically possible that they can self fertilize, however, most E. chlorotica will mate with another individual. The egg will be fertilized inside the animal, and the animal will lay fertilized eggs in long chains.
Kleptoplasty
Elysia chlorotica feeds on filamentous algae, the most well known type is Vaucheria litorea. When an E. chlorotica is eating, it punctures the cell wall with its piercing teeth. Then, it holds the algal strand and sucks up the chloroplast and other cells insides the algae. During kleptoplasty, E. chlorotica stores the chloroplasts in their digestive system and uses them for photosynthesis.
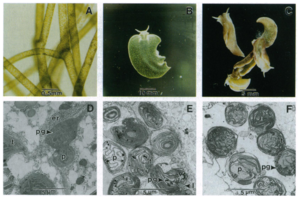
Kleptoplasty is an unusual behavior because the chloroplasts can remain temporarily functioning in the organism, which allows an animal to perform photosynthesis like plants. Recent research suggests that kleptoplasty can be obtained through lateral gene transfer from algae to the sea slug. The symbiotic chloroplasts in the organism remain functioning after 8 months’ starvation (even turning the organism yellowish Figure 3.C); and it is the longest-lived symbiosis of chloroplasts ever reported. Scientists suggest three possibilities to explain this phenomenon: (i) All plastid and nuclear-encoded chloroplast proteins remain stable in the organism, (ii) at the initial stage of symbiotic evolution, some algal nuclear genes may have been transferred into sea slug’s nuclear genome, or (iii) the symbiotic plastids can live individually and remain functioning without the algal cell nucleus and cytoplasm. By isolating E. chlorotica DNA free from interfering mucus[8], researchers concluded the algal chloroplast gene actively transcripts and translates in the sea slug. Genes such as rbcL, rbcS, psaB, psbA, and 16S rRNA have been found in the organism after months of starvation[9]. RbcL gene, which encode Rubisco LS, has been found in E. chlorotica DNA; Rubisco LS protein is also presence after two month’s starvation. PsbA Genes were remain constant until 4 months of starvation and start to decline. The 16S rRNA gene stays at a constant level from start to finish. RbcS is also remains at a steady level. These genes are directly engage in chloroplast encoding which indicates E. chlorotica can constantly encoding new symbiotic chloroplast using gene information even without food (chloroplast) source. Furthermore, the transcription and translation of chloroplast occurred in the situation where plant nucleocytoplasm is absent; which means the regulation of chloroplast gene expression is taken by sea slug’s nucleus and cytosol; or completely regulate within the symbiotic plastid genome.
Another research about E. Cholotica's encoded gene and complex provide further molecule evidence of horizontal gene transfer between multicellular eukaryotes. Three V. litorea nuclear-encoded genes [fucoxanthin chlorophyll a/c-binding protein (fcp) and light-harvesting complex 1 and 2 (Lhcv1 and 2)] had been found in sea slug's genomic DNA, primarily serve the function of support the chloroplast endosymbiosis in E. chlorotica[10].
However, chloroplast in E. Cholotica is not inheritable, the acquisition, usage and maintenance of kleptoplasty will appears on sea slug after they been feed on Vaucheria litorea.
References
- ↑ Rumpho, Mary E., Elizabeth J. Summer, Brian J. Green, Theodore C. Fox, and James R. Manhart. 2001. “Mollusc/Algal Chloroplast Symbiosis: How Can Isolated Chloroplasts Continue to Function for Months in the Cytosol of a Sea Slug in the Absence of an Algal Nucleus?” Zoology 104 (3-4): 303–12. https://doi.org/10.1078/0944-2006-00036.
- ↑ Vries, Jan de, Gregor Christa, and Sven B. Gould. 2014. “Plastid Survival in the Cytosol of Animal Cells.” Trends in Plant Science 19 (6): 347–50. https://doi.org/10.1016/j.tplants.2014.03.010.
- ↑ Garge, Suvarna. 2017. “Elysia Chlorotica (Eastern Emerald Elysia) ~ Details with Photos | Videos.” Alchetron.com. August 18, 2017. https://alchetron.com/Elysia-chlorotica#elysia-chlorotica-68323d25-2b6b-482b-b585-501377b82a5-resize-750.jpeg.
- ↑ Pierce, S. K., T. K. Maugel, M. E. Rumpho, J. J. Hanten, and W. L. Mondy. 1999. “Annual Viral Expression in a Sea Slug Population: Life Cycle Control and Symbiotic Chloroplast Maintenance.” The Biological Bulletin 197 (1): 1–6. https://doi.org/10.2307/1542990.
- ↑ Blanchet, Chelsea. 2011. “Elysia Chlorotica.” Animal Diversity Web. 2011. https://animaldiversity.org/accounts/Elysia_chlorotica/.
- ↑ “SymBio: The Slug Elysia-Development.” 2011. Web.archive.org. April 17, 2011. https://web.archive.org/web/20110417045514/http://sbe.umaine.edu/symbio/3Slug/3development.html.
- ↑ Rumpho, M. E., J. M. Worful, J. Lee, K. Kannan, M. S. Tyler, D. Bhattacharya, A. Moustafa, and J. R. Manhart. 2008. “Horizontal Gene Transfer of the Algal Nuclear Gene PsbO to the Photosynthetic Sea Slug Elysia Chlorotica.” Proceedings of the National Academy of Sciences 105 (46): 17867–71. https://doi.org/10.1073/pnas.0804968105.
- ↑ Rumpho,M.E.,Mujer,C.V.,Andrews,D.L.,Manhart,J.R.& Pierce, S. K. 1994. BioTechniques 17, 1097-1101.
- ↑ Mujer, C. V., D. L. Andrews, J. R. Manhart, S. K. Pierce, and M. E. Rumpho. 1996. “Chloroplast Genes Are Expressed during Intracellular Symbiotic Association of Vaucheria Litorea Plastids with the Sea Slug Elysia Chlorotica.” Proceedings of the National Academy of Sciences 93 (22): 12333–38. https://doi.org/10.1073/pnas.93.22.12333.
- ↑ Schwartz, Julie A., Nicholas E. Curtis, and Sidney K. Pierce. 2010. “Using Algal Transcriptome Sequences to Identify Transferred Genes in the Sea Slug, Elysia Chlorotica.” Evolutionary Biology 37 (1): 29–37. https://doi.org/10.1007/s11692-010-9079-2.
