Finegoldia magna
Classification

Domain: Bacteria
Phylum: Firmicutes
Class: Clostridia
Order: Eubacteriales
Family: Peptoniphilaceae
Genus: Finegoldia
Species
Finegoldia magna
F. magna was previously classified as Peptostreptococcus anaerobius, and it was recognized as a unique species as recently as 1998. [4]
|
NCBI: Taxonomy [5] |
Description and Significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
"Finegoldia magna" is a slow-growing, non-spore-forming, Gram-positive anaerobic coccus (GPAC) bacteria.
- F. magna is a major part of healthy skin and mucosal surface microflora. It has been shown to be the most commonly-isolated anaerobe from skin specimens [6] , and it is prevalent in chronic wounds, such as diabetic and pressure ulcers. [7] It is also commonly found as the culprit of secondary infections following prosthetic joint replacement or cardiac valve replacement surgeries. [8] ] The importance of F. magna as a human pathogen is insufficiently recognized, especially as it has been recently implicated in a case of toxic shock syndrome.
- The color of the colonies is usually translucent, but it can appear whitish to yellow or gray. [9]
- The Genus namesake is in remembrance of the American microbiologist Sydney Martin Finegold.
Genome Structure
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Cell Structure, Metabolism and Life Cycle
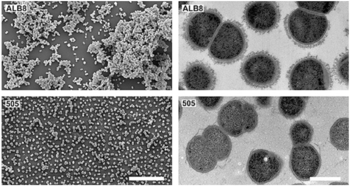
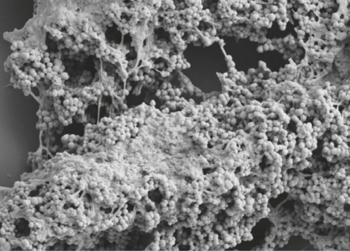
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Cell Structure
- Finegoldia magna is a Gram-positive anaerobic coccus (GPAC) bacteria, in which "coccus" indicates a spherical cell shape. Gram-positive indicates a bacterium with thick petidoglycan cell walls.
- It has a cell size that varies from 0.8 µm and 1.6 µm in diameter, and cells occur predominantly in masses or clusters but occasionally in short chains or pairs, as in Figure 4.
- This bacteria has sortase-dependent pilli, which are hair-like structures important for bacterial adhesion and colonization (i.e., important for biofilm formation) as well as host infection. [10] Sortases are enzymes that modify surface proteins of the cell wall.
Metabolism
- As an anaerobe, F. magna requires an oxygen-free environment to grow. However, in a 1998 experiment by Murdoch et al., F. magna isolates were plated on agar and exposed to air. After 48 hours, some clusters survived, indicating some level of oxygen tolerance. [11]
- F. magna produces acetic acid as a major fermentation product. It produces weak acid utilizing fructose, while a few strains use glucose as a carbon source. Peptones and amino acids are also used an energy sources. It can produce ammonia from glycine, threonine, and serine. [12]
- Aminopeptidases (i.e. catalyze cleavage of amino acids) have been reported but are not well-studied. [13]
Survival and Life Cycle
- F. magna is susceptible to antibiotics used to treat anaerobic infections. However, it shows some resistance (10-20%) to clindamycin, metronidazole, penicillin, while even higher resistance (>20%) to erythromycin and tetracycline. Its ability to form mono-species and dual-species biofilms may lend greater protection against host immune defenses and antibiotics. [1]
- The nutritional requirements of cultured GPAC organisms, including F. magna are understudied. Commercial media tend to vary in their ability to support growth. However, Fastidious Anaerobe Agar has consistently proved most successful. Sodium oleate, vitamin K, and hemin supplements are not necessary. [11]
- Cocci bacteria, including F. magna, replicate asexually through binary fission and can divide from different planes to form a variety of arrangements (as compared to bacilli, which divide from the horizontal axis).
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment. If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
References
Authors
Page authored by Susan Reed, Nitesh Naren, Matt Millikin, students of Prof. Jay Lennon at Indiana University.

