Flavivirus NS1 protein: Difference between revisions
| Line 15: | Line 15: | ||
==NS1 Structure== | ==NS1 Structure== | ||
[[Image:Alex Fig 2.jpeg|thumb|300px|left|Figure 2. NS1 dimer structure. (A) NS1 dimer with one subunit in gray | [[Image:Alex Fig 2.jpeg|thumb|300px|left|Figure 2. NS1 dimer structure. (A) NS1 dimer with one subunit in gray and the other colored by domain (blue, b roll; yellow, wing with orange connector subdomain; red, central b ladder). Disulfides are shown as yellow spheresand N-linked glycosylation sites as black sticks. A 20-residue disordered regions indicated with dotted lines. C, C terminus; N, N terminus.(Akey et al 2014). ]] | ||
<br>All flavivirus NS1 genes encode a 352 amino acid polypeptide with a slight variation in glycosylation status which gives it a variable weight of 46-55 kDa (Muller and Young 2013). Up until recently, the structure of NS1 was unknown due to its dissimilarity with known sequences and an inability to produce pure, stable proteins. Akey et al.(2014) finally elucidated the structure for the two main forms of NS1using a baclovirus expression system in insect cells which produced full length, recombinant, glycosylated West Nile and dengue type two virus NS1. In their NS1 dimer structure, all amino acids are accounted for except a twenty amino acid stretch from amino acid 108-128 (Akey et al 2014). | |||
Shown in Figure 2, the NS1 dimer is contains a central β-sheet domain with each monomer containing three domains. The first is a small β-roll domain that is a domain swap structure of two β hairpins that are stabilized with a disulfide bond. These hairpins reach across the axis and intertwine, forming a curved β-sheet as indicated in blue in figure 2A. The monomer’s second domain resembles a wing coming off of the central β-sheet. The wing, highlighted in yellow (3A), has two glycosylation sites, an internal disulfide and contains two discreet domains. One of these domains is an α/ β subdomain which is made up of a four stranded β-sheet, two α helixes, and a disordered distal tip and is represented in Fig 2A as a dotted line. The other subdomain is a discontinuous connector that sits against the β-roll and is shown in orange. NS1’s third domain is the most noticeable as it is a continuous β-sheet that runs along the whole length of the dimer and resembles a ladder. It has 18 antiparallel β strands that look like rungs with each monomer contributing exactly nine rungs. There also happens to be a long “spaghetti loop” between β13 and β14 that holds it shape through hydrogen bonds. Overall, the dimer 90 A long, has a 90A “wingspan” and is 40A in the third dimension (Akey et al. 2014). | |||
Thanks to a protrusion created by the β-roll and connector subdomain interaction, one side of the NS1 dimer is hydrophobic. Additionally, a dipeptide that has been suggested to interact with the NS4B transmembrane protein is located along the edge of the protrusion. This plus the hydrophobicity could allow the NS1 dimer to interact with the ER membrane and NS4A and B, which are a part of the replication complex along with the other six nonstructural proteins (Atkey et al. 2014). | |||
NS1 dimers are thought to be intracellular, while the other form, the hexamer, is thought to be secreted. The hexameric NS1 is a soluble, proteolipid particle that seems to have configurations that vary beween and among the West Nile and dengue viruses. Akey et al. (2014) found three different hexamer configurations but they noted that all hexamers were made up of three dimers. They also followed a trend where the three β rolls were facing the interior with the spaghetti loops, glycosylation sites, and wing domain loops were facing outward. The different configurations can be seen in Figure 3. <br> | |||
==NS1 Expression, post translational processing and trafficking == | ==NS1 Expression, post translational processing and trafficking == | ||
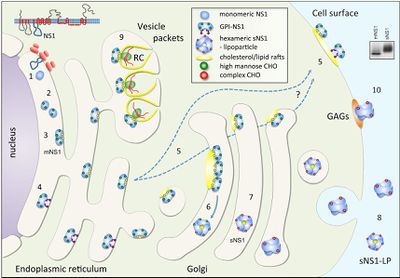
[[Image:Alex Fig 3.jpeg|thumb|400px|left|Figure 3.Schematic summary of NS1 trafficking in mammalian cells. (Muller and Young 2013) ]] | [[Image:Alex Fig 3.jpeg|thumb|400px|left|Figure 3.Schematic summary of NS1 trafficking in mammalian cells. (Muller and Young 2013) ]] | ||
Revision as of 19:08, 25 April 2014
Flavivirus
By Alex Gonzales
Flaviviruses are small, positive sense RNA viruses that can cause yellow fever, dengue, Japanese encephalitis, and West Nile virus in addition to tick-borne encephalitis (Hetnz and Stiasny 2012). These pathogens use arthropods such as mosquitoes from the Aedes genus as vectors and are found in temperate and tropical areas. The specificity of their natural hosts means that flaviviruses all have a certain endemic/ epidemic area rather than being equally distributed around the globe. However, that also means that the viruses range can expand when the host adapts to new places. For example the introduction of WN vector and the subsequent outbreak in New York in 1999 lead to the expansion of its target range into North and South America (Vasilakis et al. 2011).
Globally, the dengue virus has the most impact with about 50-100 million infections per year which result in more than 20,000 deaths annually. This is probably due to the fact that there are vaccines for yellow fever, Japanese encephalitis, and tick born encephalitis but an effective dengue vaccine has yet to be discovered. The delay in the dengue vaccine comes from concerns that the vaccines might actually predispose the subject to infections from other strains of dengue virus (Heintz, Stiasny 2012).
In the search for a viable vaccine, over three decades of research has been focused on the structure and pathology of Flaviviruses. Over the course of their work, scientists have discovered much, but many questions still remain about an enigmatic nonstructural glycoprotein, NS1. NS1 was discovered in 1970 in the serum of a patients infected with dengue and it was later discovered that all flaviviruses NS1 genes were highly homologous. Additionally, it was found that NS1 has multiple oligomeric forms a dimer and a hexamer, which are involved in replication and host immune system evasion respectively. It has also been suggested that the two main forms of NS1 are involved in both protection and pathogenesis of their host (Muller, Young 2013).
Basic Flavivirus Structure and Maturation
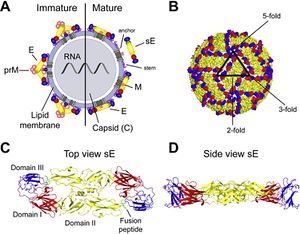
Flaviviruses are small and have only seven nonstructural and three structural proteins. These structural proteins are designated E, prM/M, and C for envelope, precursor of the membrane or membrane, and capsid respectively (Fig 1). Immature virions are formed by budding of the endoplasmic reticulum. These non-infections virions form prM-E heterodimers and in order to mature into infectious virions, these heterodimers need to be cleaved. This allows the E proteins to form herringbone like antiparallel dimers and therefore rearranges the whole viral envelope into a smoother surface (Heinz and Stiasny 2012).
E proteins are important for cell infection as they mediate binding to cell receptors and fusion with endosomal membranes after host cell endocytosis. To help fusion with the endosomal membrane, E protiens undergo yet another structural change triggered by pH. In low pH, the E protein dimer disassociates, and becomes a trimer with an exposed fusion peptide at one tip. Due to the essential role E proteins play in entering the host cell, they are a main target of anti-flivivrus antibodies which attempt to block entry into the cell by hindering attachment and endocytosis or membrane fusion (Heinz and Stiasny 2012).
NS1 Structure
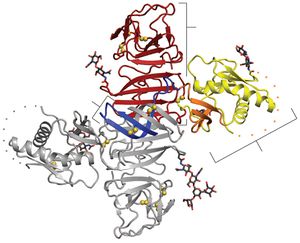
All flavivirus NS1 genes encode a 352 amino acid polypeptide with a slight variation in glycosylation status which gives it a variable weight of 46-55 kDa (Muller and Young 2013). Up until recently, the structure of NS1 was unknown due to its dissimilarity with known sequences and an inability to produce pure, stable proteins. Akey et al.(2014) finally elucidated the structure for the two main forms of NS1using a baclovirus expression system in insect cells which produced full length, recombinant, glycosylated West Nile and dengue type two virus NS1. In their NS1 dimer structure, all amino acids are accounted for except a twenty amino acid stretch from amino acid 108-128 (Akey et al 2014).
Shown in Figure 2, the NS1 dimer is contains a central β-sheet domain with each monomer containing three domains. The first is a small β-roll domain that is a domain swap structure of two β hairpins that are stabilized with a disulfide bond. These hairpins reach across the axis and intertwine, forming a curved β-sheet as indicated in blue in figure 2A. The monomer’s second domain resembles a wing coming off of the central β-sheet. The wing, highlighted in yellow (3A), has two glycosylation sites, an internal disulfide and contains two discreet domains. One of these domains is an α/ β subdomain which is made up of a four stranded β-sheet, two α helixes, and a disordered distal tip and is represented in Fig 2A as a dotted line. The other subdomain is a discontinuous connector that sits against the β-roll and is shown in orange. NS1’s third domain is the most noticeable as it is a continuous β-sheet that runs along the whole length of the dimer and resembles a ladder. It has 18 antiparallel β strands that look like rungs with each monomer contributing exactly nine rungs. There also happens to be a long “spaghetti loop” between β13 and β14 that holds it shape through hydrogen bonds. Overall, the dimer 90 A long, has a 90A “wingspan” and is 40A in the third dimension (Akey et al. 2014).
Thanks to a protrusion created by the β-roll and connector subdomain interaction, one side of the NS1 dimer is hydrophobic. Additionally, a dipeptide that has been suggested to interact with the NS4B transmembrane protein is located along the edge of the protrusion. This plus the hydrophobicity could allow the NS1 dimer to interact with the ER membrane and NS4A and B, which are a part of the replication complex along with the other six nonstructural proteins (Atkey et al. 2014).
NS1 dimers are thought to be intracellular, while the other form, the hexamer, is thought to be secreted. The hexameric NS1 is a soluble, proteolipid particle that seems to have configurations that vary beween and among the West Nile and dengue viruses. Akey et al. (2014) found three different hexamer configurations but they noted that all hexamers were made up of three dimers. They also followed a trend where the three β rolls were facing the interior with the spaghetti loops, glycosylation sites, and wing domain loops were facing outward. The different configurations can be seen in Figure 3.