Free Fatty Acids as antibacterial agents against several super pathogens including MRSA
Introduction
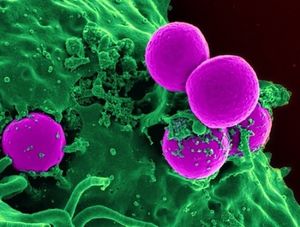
Over the past century, modern science has been overusing and misusing antibiotics, leading to the evolution of many “super pathogens”, or microbes that are resistant to most of the common antibiotics and other methods normally used to treat them. Bacteria and viruses that evade modern treatment through mutation and resistance are health dangers to hospital patients as well as community members. Staphylococcus aureus is a bacterium that colonizes the human nose and skin. It also known as an opportunistic pathogen, meaning that it can live peacefully on a body until the opportunity presents itself where it can initiate an infection in the host. However, overuse of antibiotics has led to the mutation of this pestering bacteria to the super pathogen that is MRSA, Methicillin Resistant Staphylococcus aureus . MRSA cannot be treated with an entire class of common antibiotics called beta-lactams (which include penicillin and methicillin among others) [5], and now, even the most potent “last line of defense” antibiotic, vancomycin, is ineffective on some strains of MRSA (VRSA, for vancomycin resistant Staphylococcus aureus)[6]. There are many of these pathogens - named super pathogens - that have developed resistance, including Clostridium difficile[7], several Enterococcus species including E. avium[8], along with many others.
These super pathogens demand alternate treatments from the scientific community, preferably treatments that pathogens cannot mutate around by developing resistance. Another solution might be to find treatments that could be used instead of antibiotics for non-threatening pathogens, and therefore prevent mutation/resistance in all bacteria. This would reduce the need for more potent antibiotics because super-pathogens would not evolve as quickly. A promising lead is, surprisingly, something that is already in high abundance on our own skin: fatty acids. The fatty acids found in abundance in human sebum are essential for defense against pathogens, and in healthy individuals, are a sufficient barrier to gram positive harmful bacteria [9]. Commercially, fatty acids are used to treat acne (which is also caused by bacteria) and some companies even claim they prevent aging. Other fatty acids that do not naturally occur on our skin are in many of the foods we eat, such as coconuts [10]. Surprisingly, the potential benefits to using fatty acids as antimicrobials have been hinted at and studied for decades, but they have not achieved wide usage in the medical field.
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: Ebola_virus2.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
About Free Fatty Acids
Fatty acids are a group of amphipathic biologically available compounds comprised of a carboxylic acid and a hydrophobic tail of varying carbon chain lengths. If there are any double bonds in the carbon chain, the molecule is an unsaturated fatty acid, and the number of double bonds in the carbon chain determines how unsaturated it is. If there are no double bonds, then the fatty acid is considered saturated. The “free” part of the term free fatty acid, just means that it is not attached to any other compound in the cell. Fatty acids are found in many biological systems, including the human system. They are considered macronutrients, along with proteins and carbohydrates, and they yield the most ATP per gram out of all three. When fatty acids are used for energy storage in the body, they are stored as triglycerides, which are molecules containing three fatty acids attached to a glycerol molecule. When we eat fats, we normally are ingesting triglycerides. [11]
Another very important aspect of fatty acids in the body is cell membrane phospholipids, which are composed of two fatty acids attached by a phosphate group. The cell membrane is one of the primary characteristics of life. Some fatty acids even act as important signaling molecules, different from others because they can passively diffuse through hydrophobic membranes.[12] There are so many diverse uses for fatty acids in the human body, but what tends to get overlooked, is their role in epidermal defense. Yes, there are tons of fatty acids, some that we produce ourselves, and some that we need to ingest, that we come into contact with every day. They are on our skin, protecting us from external factors like microbes, but only the ones in their carboxylic acid forms.[13]
Fatty acids that are present in the epidermis, specifically in the stratum corneum, the outermost layer of the skin, consist primarily of palmitic acid, stearic acid, palmitoleic acid, oleic acid, linoleic acid (an essential fatty acid), and (cis)-11,14,17-eicosatrienoic acid (ETA).[14] However, epidermal fatty acid types and amounts vary among individuals, and do not change according to environmental factors, indicating that the type and amount produced is controlled by genetics.[15] Humans do not synthesize linoleic or linolenic acid, and therefore must be consumed in the diet. Since fatty acids play an important role in the protection of the body, these differences may be a reason why some people are more susceptible to skin disease or infection.
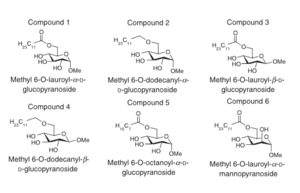
History of Fatty Acids as Antimicrobial Agents
Scientists have known about acids as anti-fungal products since 1899, when the “Toxic Effect of Deleterious Agents” was seen on fungi development.[16] Slightly later, in 1948, a review of “surface active agents” noted that quaternary ammonium salts increased their bactericidal activity with longer chain lengths.[17] Anionic compounds (fatty acids fit into this category) were shown to have activity only against gram-positive organisms. The toxicity of these quaternary agents affixed with groups such as chlorine, mercury, permanganate, alcohol, or formadehyde, with the least toxic being ammonium. We know now that these groups generally toxic to animal tissues, and more recent research has been looking at these fatty acids that are found in large quantities in other living systems (including our own).
Hydrolyzed fatty acids seem to have a higher antimicrobial effect than un-hydrolyzed fatty acids. An example of this is seen with virgin coconut oil, where the hydrolyzed coconut oil had antibacterial activity, but the unhydrolyzed oil did not. With increased addition of NaOH, antimicrobial activity increased, suggesting that the formation of the hydrolyzed coconut oil was what was antimicrobial.[18]
In 1972 a research group from Michigan Sate University published extensive data on the effects of several fatty acids on both gram-positive and gram negative bacteria.[19] They demonstrated that by increasing the unsaturation of the fatty acids through the addition of cis double bonds, they could make these fatty acids more effective against gram-positive bacteria, but when the carboxylic acid was esterified the compounds lost their antibacterial effect. Strangely, they found that lauric acid, a saturated fatty acid, had the highest bacteriostatic potential towards these gram-positive bacteria of which included Staphylococcus genus, (which includes MRSA). Lauric acid was found to have the highest antimicrobial activity out a pool of saturated fatty acids, but overall, saturated fatty acids had lower activity than those that were unsaturated.[20] Palmitoleic acid, a monoene of human skin, was found to be most active against gram-positive bacteria, although in combination with ethanol, a synergistic effect against gram-negative bacteria (P. aeruginosa, P. acnes, E. coli) was seen.
Staphylococci epidermidis are found in abundance on healthy skin, whereas S. aureus are usually only found on damaged skin, but the reasons for this are unknown. A study in 1985 showed that staphylococci that were coagulase-positive, i.e. S. aureus, on the skin were sensitive to linolenic acid,[21] while other fatty acids were determined to have the same activity against coagulase-positive versus coagulase-negative staphylococci suggesting interference with the enzyme coagulase as a possible mechanism of action. This finding supports the premis that treatment with linolenic acid may be species specific, which is important because preserving staphylococcus not of the species aureus is beneficial to the human system. The researchers even mutated the S. aureus, changing the characteristics of the bacteria in an attempt to mimic rapid evolution associated with resistance, to find a resistant strain towards linoleic acid. They were unsuccessful, suggesting linolenic acid will not cause resistance in bacteria, like other forms of treatment such as antibiotics. They also found that if human skin was covered in linolenic acid prior to the seeding of S. aureus, the bacteria were rapidly killed. However, the same group also found that antibacterial effects of this acid were reversed in vitro when low concentrations of human serum were present. They suggest a potential “detergent” mechanism, possibly the change in the functional group of the acid. This suggests potential problems with internal treatment of bacterial infections with linolenic acid. Another group got similar result with a different fatty acid, lauric acid, where human plasma decreased its antimicrobial activity.[22]
Bactericidal Effects of Fatty Acids on Methicillin Resistant and Susceptible Staphylococcus aureus
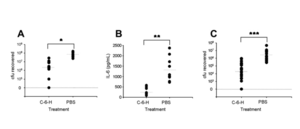
Include some current research, with at least one figure showing data.
Conclusion
References
[1] Wikimedia. NIH.
[5] http://www.niaid.nih.gov/topics/antimicrobialResistance/Examples/mrsa/
[6] Gould, IM. 2010. VRSA-doomsday superbug or damp squib? Lancet Infect Dis 10 (12): 816–8. doi:10.1016/S1473-3099(10)70259-0. PMID 21109164.
[7] http://www.cdc.gov/drugresistance/threat-report-2013/index.html
[8] http://www.antimicrobe.org/new/b03.asp
[9] Clarke, S. R., R. Mohamed, L. Bian, A. F. Routh, J. Kokai-Kun, J. J. Mond, A. Tarkowski, and S. J. Foster. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1:199-212
[10] Verallo‐Rowell, V.M., Dillague, K.; Syah‐Tjundawan, B.S. 2008. Novel Antibacterial and Emollient Effects of Coconut and Virgin Olive Oils in Adult Atopic Dermatitis. Journal of Dermatitis. 19 (6):301-358
[11] https://en.wikipedia.org/wiki/Fatty_acid
[12] http://www.nature.com/scitable/topicpage/fatty-acid-molecules-a-role-in-cell-14231940
[13] Butcher GW, King G, Dyke KG. 1976. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J. Gen. Microbiol. 94:290 –296. http://dx .doi.org/10.1099/00221287-94-2-290.
[14] Kim, E. J., Kim, M.-K., Jin, X.-J., Oh, J.-H., Kim, J. E., & Chung, J. H. (2010). Skin Aging and Photoaging Alter Fatty Acids Composition, Including 11,14,17-eicosatrienoic Acid, in the Epidermis of Human Skin. Journal of Korean Medical Science, 25(6), 980–983. http://doi.org/10.3346/jkms.2010.25.6.980
[15] Green, S.C.S, M.E., Downing, D.T. (1984). Variation in Sebum Fatty Acid Composition Among Adult Humans. 1984 J Investig Dermatol. 83 (2): 114-117 http://dx.doi.org/10.1111/1523-1747.ep12263287
[16] Clark, J. R. 1899. On the toxic effect of deleterious agents on the germination and development cf certain filamentous fungi. Botan. Gaz. 28:289-327.
[17] Glassman, H. N. 1948. Surface active agents and their application in bacteriology. Bacteriol.Rev. 12:105-148
[18] http://innovareacademics.in/journals/index.php/ajpcr/article/view/1042
[19] Kabara, J. J., Swieczkowski, D. M., Conley, A. J., & Truant, J. P. (1972). Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrobial Agents and Chemotherapy, 2(1), 23–28.
[20] Wille J, J, Kydonieus A, Palmitoleic Acid Isomer (C16:1Δ6) in Human Skin Sebum Is Effective against Gram-Positive Bacteria. Skin Pharmacol Physiol 2003;16:176-187
[21] Lacey, R., Lord V.J. Med. Microbiol. 14(1):41-49 doi:10.1099/00222615-14-1-41
Authored for BIOL 291.00 Health Service and Biomedical Analysis, taught by Joan Slonczewski, 2016, Kenyon College.
