Glycylcycline Antibiotics: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
Currently tigecycline (previously GAR-936) is the only antibiotic of the glycylcycline class in clinical use. Tigecycline was approved by the Food and Drug Administration in 2005, and is particularly useful in the treatment of multi-drug resistant infections, which are especially hard to treat. Penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis, and vancomycin-resistant Enterococcus species are several examples of species that have developed an antibiotic resistance but are still affected by tigecycline. In addition to these, there is a wide variety of organisms, including both gram-positive and gram-negative bacteria, that are sensitive to tigecycline. It does not, however, seem to have any effect of Proteus, Providencia, or Pseudomons species. The antibiotic is structurally very similar to minocycline and similarly binds to the bacterial 30S ribosome unit. The manner in which the molecule binds prevents amino-acyl tRNAs from binding to the A site of the ribosome and subsequently prevents peptide formation and bacterial growth. Furthermore, the main difference between tigecycline and minocycline is the addition of an N,N-dimethylglycylamido group which actually causes the molecule to bind to the ribosome up to five times more tightly and decreases the probability that resistance will develop. <br><br> | Currently tigecycline (previously GAR-936) is the only antibiotic of the glycylcycline class in clinical use. Tigecycline was approved by the Food and Drug Administration in 2005, and is particularly useful in the treatment of multi-drug resistant infections, which are especially hard to treat. Penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis, and vancomycin-resistant Enterococcus species are several examples of species that have developed an antibiotic resistance but are still affected by tigecycline. In addition to these, there is a wide variety of organisms, including both gram-positive and gram-negative bacteria, that are sensitive to tigecycline. It does not, however, seem to have any effect of Proteus, Providencia, or Pseudomons species. The antibiotic is structurally very similar to minocycline and similarly binds to the bacterial 30S ribosome unit. The manner in which the molecule binds prevents amino-acyl tRNAs from binding to the A site of the ribosome and subsequently prevents peptide formation and bacterial growth. Furthermore, the main difference between tigecycline and minocycline is the addition of an N,N-dimethylglycylamido group which actually causes the molecule to bind to the ribosome up to five times more tightly and decreases the probability that resistance will develop. <br><br> | ||
The glycylcycline class of antibiotics is characterized by a molecular structure containing a four-ring carbocyclic skeleton with a substitution of an N-alkylglycylamido group at the D-9 position. Biochemical experiments have shown that the tigecycline binds to the same site on 16S rRNA as tetracycline but in a different orientation and with greater affinity. This has been confirmed by experiments in which a decreased binding affinity was observed in strains of E. coli with known mutations in the A site in the rRNA (G966U or G1058C). <br><br> | The glycylcycline class of antibiotics is characterized by a molecular structure containing a four-ring carbocyclic skeleton with a substitution of an N-alkylglycylamido group at the D-9 position. Biochemical experiments have shown that the tigecycline binds to the same site on 16S rRNA as tetracycline but in a different orientation and with greater affinity. This has been confirmed by experiments in which a decreased binding affinity was observed in strains of E. coli with known mutations in the A site in the rRNA (G966U or G1058C). <br><br> | ||
Tigecycline is administered to a patient intravenously with a dose of 50 mg every 12 hours after an initial 100 mg loading dose. As with nearly all drugs, the antibiotic has several known side-effects, including nausea, vomiting, and diarrhea, but these are relatively minor. Barring severe complications, the treatment period usually ranges from five to fourteen days. | Tigecycline is administered to a patient intravenously with a dose of 50 mg every 12 hours after an initial 100 mg loading dose. As with nearly all drugs, the antibiotic has several known side-effects, including nausea, vomiting, and diarrhea, but these are relatively minor. Barring any severe complications, the treatment period usually ranges from five to fourteen days. <br><br> | ||
<br> | <br> | ||
==Comparison of Tetracycline and Tigecycline Ribosome Binding== | ==Comparison of Tetracycline and Tigecycline Ribosome Binding== | ||
<br>Bauer et al. have identified the binding sites of tetracycline and tegecycline through probing 70S E. coli ribosomes with dimethylsulphate (DMS) and Fe2+-mediated cleavage. This method works by allowing Fe2+ to replace Mg2+ complexed with tetracyclines. When H2O2 is added, the Fe2+ produces transient, very reactive hydroxy radicals that can cleave RNA close to where tetracycline is bound. It follows then, that the resulting cleavage sites will also be the location at which tetracycline binds to the ribosome. Crystallography experiments have shown that there are six tetracycline binding sites on the 30S ribosome. <br><br> | <br> Bauer et al. have identified the binding sites of tetracycline and tegecycline through probing 70S E. coli ribosomes with dimethylsulphate (DMS) and Fe2+-mediated cleavage. This method works by allowing Fe2+ to replace Mg2+ complexed with tetracyclines. When H2O2 is added, the Fe2+ produces transient, very reactive hydroxy radicals that can cleave RNA close to where tetracycline is bound. It follows then, that the resulting cleavage sites will also be the location at which tetracycline binds to the ribosome. Crystallography experiments have shown that there are six tetracycline binding sites on the 30S ribosome. <br><br> | ||
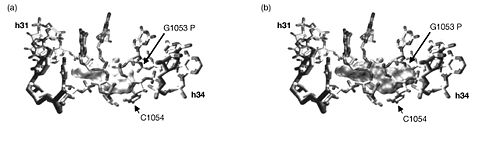
It was found that three sites were cleaved in the rRNA in the presence of tetracycline. Two of the three sites, those in h31 and h34, corresponded with tetracycline binding site-1. These were confirmed through a point mutation (G1058 to C) in h34 that produced tetracycline resistance. Additional mutation experiments showed that mutations in these areas also resulted in an eight-fold increase in resistance to tigecycline. The third rRNA cleavage site in h29 corresponds to tetracycline binding site-4, so the observation is confirmed by crystallography data. There is, however, no evidence of a magnesium ion complexed with tetracycline at tetracycline binding site-2. It follows, then, that the Fe2+-mediated cleavage technique would not suggest a tetracycline at this site. The other tetracycline binding sites have previously shown no evidence of being involved in antibiotic activity. <br><br> | It was found that three sites were cleaved in the rRNA in the presence of tetracycline. Two of the three sites, those in h31 and h34, corresponded with tetracycline binding site-1. These were confirmed through a point mutation (G1058 to C) in h34 that produced tetracycline resistance. Additional mutation experiments showed that mutations in these areas also resulted in an eight-fold increase in resistance to tigecycline. The third rRNA cleavage site in h29 corresponds to tetracycline binding site-4, so the observation is confirmed by crystallography data. There is, however, no evidence of a magnesium ion complexed with tetracycline at tetracycline binding site-2. It follows, then, that the Fe2+-mediated cleavage technique would not suggest a tetracycline at this site. The other tetracycline binding sites have previously shown no evidence of being involved in antibiotic activity. <br><br> [[Image:16sbinding.jpeg|thumb|500px|right|Surface structures of tetracycline (a) and glycylcycline derivative DMG-DMDOT (b) bound to site-1. The bulky substituent on DMG-DMDOT overlaps with the phosphate backbone and prevents the molecule from binding in the same orientation that tetracycline does.]] | ||
With this knowledge, the authors conclude that since the three cleavage sites correspond to the physiologically important tetracycline binding sites and since the experiments produced identical results whether tetracycline or tigecycline was used, then tetracycline and tigecycline must share the same binding sites on 16S rRNA. Furthermore, tigecycline appears to bind much more tightly and at lower concentrations. There were, however, some differences in the Fe2+ cleavages intensities between tetracycline and tigecyclince. So although they do bind the ribosome in the same location, tigecycline likely binds in a different orientation than does tetracycline. The authors propose that the bulky substituent at position 9 on tigecycline but lacking on tetracycline is responsible for this difference. | With this knowledge, the authors conclude that since the three cleavage sites correspond to the physiologically important tetracycline binding sites and since the experiments produced identical results whether tetracycline or tigecycline was used, then tetracycline and tigecycline must share the same binding sites on 16S rRNA. Furthermore, tigecycline appears to bind much more tightly and at lower concentrations. There were, however, some differences in the Fe2+ cleavages intensities between tetracycline and tigecyclince. So although they do bind the ribosome in the same location, tigecycline likely binds in a different orientation than does tetracycline. The authors propose that the bulky t-butylglycylamido substituent at position 9 on tigecycline but lacking on tetracycline is responsible for this difference. To further investigate this, they compare the superimposed co-crystal structures of tetracycline and DMG-DMDOT (a glycylcycline derivative very similar to tigecycline) complexed with helices 31 and 34, which make up tetracycline binding site-1. This comparison revealed that the bulky dimethylglycylamido substituent on DMG-DMOT would clash with the phosphate backbone of G1053, forcing it to bind the ribosome differently. Likewise, the t-butylglycylamido substituent on tigecycline is even bigger and would presumably change the orientation in which tigecycline binds in a similar, if not more pronounced, way. <br><br> | ||
<br> | <br> | ||
Revision as of 23:40, 15 April 2009
Introduction
Kristina Buschur
The ability of bacteria to quickly develop resistance to commonly used antibiotics is a huge hurdle in the path of disease treatment. Because of this, there is an ever-present need to develop new antibiotics that are use novel mechanisms to overcome multidrug-resistance and prevent microbial growth. The glycylcycline class of antibiotics is such one recently-developed tool to combat this problem. Derived from tetracycline, glycylcyclines have added substituents that interfere with the mechanisms bacteria employ to resist tetracycline, such as tetracycline-specific efflux pumps and ribosomal protection proteins. Since tetracycline has been in wide use since the mid-1900s for treatment of many human and animal infections and as growth promoters in agriculture, many bacteria have since developed these mechanisms to prevent the harmful effects of tetracycline.
Currently tigecycline (previously GAR-936) is the only antibiotic of the glycylcycline class in clinical use. Tigecycline was approved by the Food and Drug Administration in 2005, and is particularly useful in the treatment of multi-drug resistant infections, which are especially hard to treat. Penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis, and vancomycin-resistant Enterococcus species are several examples of species that have developed an antibiotic resistance but are still affected by tigecycline. In addition to these, there is a wide variety of organisms, including both gram-positive and gram-negative bacteria, that are sensitive to tigecycline. It does not, however, seem to have any effect of Proteus, Providencia, or Pseudomons species. The antibiotic is structurally very similar to minocycline and similarly binds to the bacterial 30S ribosome unit. The manner in which the molecule binds prevents amino-acyl tRNAs from binding to the A site of the ribosome and subsequently prevents peptide formation and bacterial growth. Furthermore, the main difference between tigecycline and minocycline is the addition of an N,N-dimethylglycylamido group which actually causes the molecule to bind to the ribosome up to five times more tightly and decreases the probability that resistance will develop.
The glycylcycline class of antibiotics is characterized by a molecular structure containing a four-ring carbocyclic skeleton with a substitution of an N-alkylglycylamido group at the D-9 position. Biochemical experiments have shown that the tigecycline binds to the same site on 16S rRNA as tetracycline but in a different orientation and with greater affinity. This has been confirmed by experiments in which a decreased binding affinity was observed in strains of E. coli with known mutations in the A site in the rRNA (G966U or G1058C).
Tigecycline is administered to a patient intravenously with a dose of 50 mg every 12 hours after an initial 100 mg loading dose. As with nearly all drugs, the antibiotic has several known side-effects, including nausea, vomiting, and diarrhea, but these are relatively minor. Barring any severe complications, the treatment period usually ranges from five to fourteen days.
Comparison of Tetracycline and Tigecycline Ribosome Binding
Bauer et al. have identified the binding sites of tetracycline and tegecycline through probing 70S E. coli ribosomes with dimethylsulphate (DMS) and Fe2+-mediated cleavage. This method works by allowing Fe2+ to replace Mg2+ complexed with tetracyclines. When H2O2 is added, the Fe2+ produces transient, very reactive hydroxy radicals that can cleave RNA close to where tetracycline is bound. It follows then, that the resulting cleavage sites will also be the location at which tetracycline binds to the ribosome. Crystallography experiments have shown that there are six tetracycline binding sites on the 30S ribosome.
It was found that three sites were cleaved in the rRNA in the presence of tetracycline. Two of the three sites, those in h31 and h34, corresponded with tetracycline binding site-1. These were confirmed through a point mutation (G1058 to C) in h34 that produced tetracycline resistance. Additional mutation experiments showed that mutations in these areas also resulted in an eight-fold increase in resistance to tigecycline. The third rRNA cleavage site in h29 corresponds to tetracycline binding site-4, so the observation is confirmed by crystallography data. There is, however, no evidence of a magnesium ion complexed with tetracycline at tetracycline binding site-2. It follows, then, that the Fe2+-mediated cleavage technique would not suggest a tetracycline at this site. The other tetracycline binding sites have previously shown no evidence of being involved in antibiotic activity.
With this knowledge, the authors conclude that since the three cleavage sites correspond to the physiologically important tetracycline binding sites and since the experiments produced identical results whether tetracycline or tigecycline was used, then tetracycline and tigecycline must share the same binding sites on 16S rRNA. Furthermore, tigecycline appears to bind much more tightly and at lower concentrations. There were, however, some differences in the Fe2+ cleavages intensities between tetracycline and tigecyclince. So although they do bind the ribosome in the same location, tigecycline likely binds in a different orientation than does tetracycline. The authors propose that the bulky t-butylglycylamido substituent at position 9 on tigecycline but lacking on tetracycline is responsible for this difference. To further investigate this, they compare the superimposed co-crystal structures of tetracycline and DMG-DMDOT (a glycylcycline derivative very similar to tigecycline) complexed with helices 31 and 34, which make up tetracycline binding site-1. This comparison revealed that the bulky dimethylglycylamido substituent on DMG-DMOT would clash with the phosphate backbone of G1053, forcing it to bind the ribosome differently. Likewise, the t-butylglycylamido substituent on tigecycline is even bigger and would presumably change the orientation in which tigecycline binds in a similar, if not more pronounced, way.
Antibacterial Activity of Tigecycline
Include some current research in each topic, with at least one figure showing data.
Section 3
Include some current research in each topic, with at least one figure showing data.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.