HPV Virology and Treatment: Difference between revisions
Chobaniank (talk | contribs) |
Chobaniank (talk | contribs) |
||
| Line 18: | Line 18: | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
Virology/Oncogenesis/Molecular detection | |||
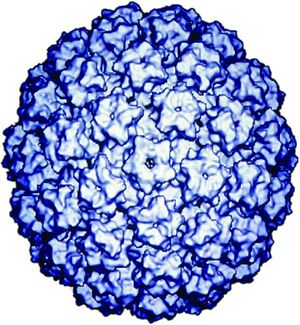
HPV is a double-stranded DNA, non-enveloped capsid virus. It has 7900 base pairs which have 90% homology between the types (1). The base pairs are arranged in a circle which includes the codes for two key proteins known as L1 and L2 (2). These two proteins act as the “immunogene” which is required for self-assembly and the infectivity protein, respectively (3,4). The virus is transmitted between humans through breaks within the epidermis of the skin. Once the virus enters the skin, it attaches to a component of skin stem cells known as the tissue-specific heparin sulfate proteoglycans (5,6, 7). Differentiation of the virus then occurs within the squamous keratinocyte (8), replicates, proliferates, and then moves to the next cell. It is the persistence of HPV within the squamous which poses the greatest risk for malignant degeneration. [See the figure below from Robert W. Trindle, Nature Reviews Cancer 2:59-64, 2002] | |||
HPV types are differentiated by their associations with specific types of human cancers. For example, HPV 16 and 18 are most frequently associated with cervical cancer (9). HPV 16 accounts for up to 50% of HPV causing cervical and anal cancers worldwide (9,10,11). In addition of those viruses associated with penile cancer, 70% are of the HPV 16 phenotype and occurs 6 times higher in males with histories of venereal warts or condylomata accuminata. | |||
HPVs have been proven to be the causative agents of other epithelial cancers. The proof that these viruses indeed are the causative agents is based upon the following observations (12): | |||
1)HPV DNA has been found within cancer biopsies | |||
2)known viral oncogenes E6 and E7 are found in cancer material | |||
3)genes E6 and E7 code for proteins which are growth-regulators in host cells | |||
4)E6 and E7 gene expression have been identified in cervical cancer immortalized cell lines | |||
5) epidemiologic investigations have identified HPV as a major factor for the development of cervical cancer | |||
6) species specific papillomaviruses cause cancer in experimental animal models including the rabbit and cow; and human neonatal foreskin infected with HPV-16 and placed in severe combined immunodeficient mice form intraepithelial neoplasms (Mandel) | |||
Molecular pathogenesis of skin cancer implicates HPV-E6 gene product binding to the human p53 tumor suppressor protein as the initial event (13-15). The p53 protein is a negative regulator the cell cycle transformation step between G0/G1 to the S phase. By degrading the p53 protein cell growth is directly impeded. E7 appears to exert an anti-cell death (anti-apoptotic) effect in cells through mutations in the p53 protein (16,17) by interfering with the G1 or arrest phase of the cell cycle. Thus, both genes negatively impact normal cell cycles causing an unregulated, unimpeded cell growth, i.e. cancer transformation. | |||
HPV is now generally detected in human tissue by various molecular biologic techniques including in situ hybridization (ISH), Southern transfer hybridization (STH), hybrid capture (HC), dot blot (DB), filter hybridization (FH), or polymerase chain reaction (PCR) (18). ISH is sensitive and can be performed directly on infected tissue specimens. It is both tedious and less sensitive than PCR and HC and thus rarely used. DB, FH, and STH are reserved for research studies and are capable of providing separations among the HPV types. HC requires a long single-stranded RNA probe to hybridize with a whole HPV genome in solution. This test enables identification of specific HPVs and quantifies them. It currently is the only method approved by the Federal Drug Administration in the United States for detection of HPV in cervical samples. PCR remains the most sensitive method for detection. It utilizes a specific primer sequence for a specific type of HPV which as a result can be used to verify links between the virus and various types of human cancers. | |||
==Sample Section 2== | ==Sample Section 2== | ||
Revision as of 22:46, 2 December 2009
Introduction
The Human Papillomarvirus or frequently referred to as HPV currently remains the most common sexually transmitted disease in the United States. Human papillomavirus (HPV) or the “wart virus” is a virus belonging to its own family, papillomaviridae. There are more than a 100 types of HPV and as reflected in its name, species specific for humans. There are two types, cutaneous or mucocutaneous, based upon its tissue tropism or predilection for infecting specific areas of the human body. Human papillomavirus infections are diagnosed worldwide, account for the most common sexually transmitted disease worldwide, and have been associated with both cutaneous and mucocutaneous cancers prompting attempts to prevent infections using vaccines. In short, it has become a global health infection warranting every effort to control and eradicate it.
To upload an image: Use "Upload File" (in margin at left)
To make the image appear, you need to embed image insertion code (see sample at right). The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Virology/Oncogenesis/Molecular detection
HPV is a double-stranded DNA, non-enveloped capsid virus. It has 7900 base pairs which have 90% homology between the types (1). The base pairs are arranged in a circle which includes the codes for two key proteins known as L1 and L2 (2). These two proteins act as the “immunogene” which is required for self-assembly and the infectivity protein, respectively (3,4). The virus is transmitted between humans through breaks within the epidermis of the skin. Once the virus enters the skin, it attaches to a component of skin stem cells known as the tissue-specific heparin sulfate proteoglycans (5,6, 7). Differentiation of the virus then occurs within the squamous keratinocyte (8), replicates, proliferates, and then moves to the next cell. It is the persistence of HPV within the squamous which poses the greatest risk for malignant degeneration. [See the figure below from Robert W. Trindle, Nature Reviews Cancer 2:59-64, 2002]
HPV types are differentiated by their associations with specific types of human cancers. For example, HPV 16 and 18 are most frequently associated with cervical cancer (9). HPV 16 accounts for up to 50% of HPV causing cervical and anal cancers worldwide (9,10,11). In addition of those viruses associated with penile cancer, 70% are of the HPV 16 phenotype and occurs 6 times higher in males with histories of venereal warts or condylomata accuminata.
HPVs have been proven to be the causative agents of other epithelial cancers. The proof that these viruses indeed are the causative agents is based upon the following observations (12):
1)HPV DNA has been found within cancer biopsies 2)known viral oncogenes E6 and E7 are found in cancer material 3)genes E6 and E7 code for proteins which are growth-regulators in host cells 4)E6 and E7 gene expression have been identified in cervical cancer immortalized cell lines 5) epidemiologic investigations have identified HPV as a major factor for the development of cervical cancer 6) species specific papillomaviruses cause cancer in experimental animal models including the rabbit and cow; and human neonatal foreskin infected with HPV-16 and placed in severe combined immunodeficient mice form intraepithelial neoplasms (Mandel)
Molecular pathogenesis of skin cancer implicates HPV-E6 gene product binding to the human p53 tumor suppressor protein as the initial event (13-15). The p53 protein is a negative regulator the cell cycle transformation step between G0/G1 to the S phase. By degrading the p53 protein cell growth is directly impeded. E7 appears to exert an anti-cell death (anti-apoptotic) effect in cells through mutations in the p53 protein (16,17) by interfering with the G1 or arrest phase of the cell cycle. Thus, both genes negatively impact normal cell cycles causing an unregulated, unimpeded cell growth, i.e. cancer transformation.
HPV is now generally detected in human tissue by various molecular biologic techniques including in situ hybridization (ISH), Southern transfer hybridization (STH), hybrid capture (HC), dot blot (DB), filter hybridization (FH), or polymerase chain reaction (PCR) (18). ISH is sensitive and can be performed directly on infected tissue specimens. It is both tedious and less sensitive than PCR and HC and thus rarely used. DB, FH, and STH are reserved for research studies and are capable of providing separations among the HPV types. HC requires a long single-stranded RNA probe to hybridize with a whole HPV genome in solution. This test enables identification of specific HPVs and quantifies them. It currently is the only method approved by the Federal Drug Administration in the United States for detection of HPV in cervical samples. PCR remains the most sensitive method for detection. It utilizes a specific primer sequence for a specific type of HPV which as a result can be used to verify links between the virus and various types of human cancers.
Sample Section 2
Include some current research in each section.
Sample Section 3
Include some current research in each section.
Conclusion
Overall paper length should be approximately 2,000 to 2,500 words.
Include at least two data figures.
Use professional sources, including at least two research studies.
Public Health Measures Against HPV
References
Edited by student of Joan Slonczewski for BIOL 191 Microbiology, 2009, Kenyon College.