Hydrothermal vents
Physical Environment

Hydrothermal vents are where fluids flow from rock fractures in the sea floor at a variety of depths, and are mainly present near volcanic and tectonic plate activity [1][2] [3]. This results in hydrothermal vents to primarily be located along ocean ridges (Fig. 1). Tectonic activity, cooling of newly formed crust, and presence of magma chambers causes convective circulation of cold seawater into the lithosphere where it is heated and enriched with nutrients such as iron and hydrogen sulfide [4]. The hydrothermal fluids mainly contain hydrogen sulfide, methane, and heavy metals, resulting in chemotrophy being the sole process for energy at depths where light is absent [2] [3]. This superheated and nutrified seawater then permeates through vents along seafloor spreading centers. Vents are characterized as one of three general types: diffuse flow, white smokers and black smokers. Diffuse vents emit low temperature (30 C), clear water. White smokers emit milky fluid approximately 200-300 C. Black smokers eject superheated (300-400 C) fluid consisting of sulfide precipitates [4]. The extreme environment found here includes pressures up to 420 atm, chemical toxicity , and fluctuating temperatures from a low of 2°C of surrounding seawater to a high of 400°C, [5]. While active vents may exist for years or even decades, the environmental characteristics such as temperature, salinity, or nutrients may fluctuate over several different time scales, ranging from seconds to years. [4] [7]. Extremely dynamic thermal and chemical gradients at vents cause segregation and limitation of organism niches over space and time [4].
Biological Interactions
Many invertebrate animals have adapted to the environment of hydrothermal vents by creating symbiotic relationships with bacteria [3]. One organism frequently studied is Riftia pachyptila, a giant tubeworm [8]. R. pachptila lack a gut and rely on bacterial symbionts for their nutrition. R. pachptila have an extensively vascularized red “plume” which extends into the water column and absorbs oxygen, hydrogen sulfide, and carbon dioxide, which the bacterial symbionts use for growth. These chemicals are transported from the plume to the trophosome where the bacterial symbionts are contained [8] [9]. Carbon dioxide is fixed by bacterial symbionts through the Calvin cycle and then organic carbon molecules are transferred from the symbiont to the host, serving as the major food source for the host [8].
The mussel Bathymodiolus azoricus also has bacterial symbionts[3]. The mussel’s gills and digestive system are locations of heavy metal accumulation [3]. Non-symbiotic free-living bacteria at hydrothermal tal resistance, with one mechanism involving heavy metal precipitation on the cell wall [3]. A similar mechanism may occur in bacterial endosymbionts in the mussel [3]. This wide array of resistant bacteria opens allows multiple bacteria to form endosymbiotic relationships with B. azoricus. Evidence shows that the bacterial endosymbionts in the gill of B. azoricus are not homogeneous [10]. The assemblage in the gill contains two different Gammaproteobacteria morphotypes. They are either sulfur-oxidizing and methane-oxidizing [11]. Although dual-symbiosis is present in B. azoricus, environmental conditions may determine the relative abundance of each morphotype based on chemical composition. This may result in an increase of available habitat due to plasticity in chemicals utilized to gain energy [10]. Dual-symbiosis is also present in other deep-sea mytelid mussels.
Alvinella pompejana, commonly known as the Pompeii worm, is an annelid polychaete that experiences a unique form of bacterial symbiosis. The Pompeii worm is an extremeophile that inhabits high temperature black smokers in the Pacific, and has external, rather than internal, bacterial symbionts [12]. The epibionts form filamentous “hairs” along the dorsal region of the worm and are fed by a mucus secretion. These epibiont communities are extremely diverse, but most of their biomass is contributed by epsilonproteobacteria [12]. It is thought that these bacterial communities may serve as a food source that is both stable and nutritious [13].
Rimicaris exoculata, or the vent shrimp, inhabits vent chimney walls where temperatures range from 10-30 C, and whose gill chamber acts as an ever changing microbial biome. Living in such close proximity to chimney vents, the vent shrimp's exoskeletal elements become encrusted by mineral deposits which results in the need to molt frequently. These mineral deposits form a nutrient rich environment for a suite of symbiotic bacteria, including gammaproteobacteria, epsilonproteobacteria, and zetaproteobacteria. However, the frequent molting results in a continual re-colonization and competition between these bacterial types. These epibionts utilize the mineral deposits on the exoskeleton on the vent shrimp as an energy source while the vent shrimp presumably consumes the bacteria for nutritional benefit [14].
Calyptogena magnifica, or the hydrothermal vent clam, rely on endosymbiotic bacteria as their primary source of nourishment which can be acquired by vertical and horizontal transmission [15]. Hydrothermal vent clams have a reduced gut and food groove which cause them to be entirely dependent on these endosymbionts [16]. These gammaproteobacteria, Candidatus Ruthia magnifica, oxidize sulfur that is brought in by the clam's intake siphon, and are then consumed by the clam. These gammaproteobacteria genomes encode for metabolic pathways that are typical for free-living bacteria, but culturing techniques have not resulted in successful isolation of these bacteria. Vertical transmission of these endosymbionts has been found in cytosolic elements. However, these endosymbionts may be horizontally transmitted between clams, or may even change hosts within each clam species [15].
Hydrothermal vent squat lobsters (Genus: Kiwa), or better known as the Yeti crab, are present on black smokers and mussel beds along the Pacific Antarctic Ridge. They are covered in dense patches of setae. These setae cover the legs and the ventral surface of the cephalothorax and are colonized by filamentous bacteria. These bacterial communities are composed of gammaproteobacteria, epsilonproteobacteria, and Bacteriodetes [17]. The function of these bacterial communities are relatively unknown, but some evidence has been presented for the potential benefits. These bacterial communities may help protect the host from predators and the environment, mainly as embryos or larvae. These bacterial communities also oxidize sulfur and may provide nourishment for the Yeti crab [17].
Microbial Processes
Chemoautotrophy happens by at least two pathways, the reverse TCA cycle, which is more prominent, and the Calvin cycle [7]. The reverse TCA cycle uses four CO2 to make one oxaloacetate, consuming four to five ATPs in the process [7]. This energy requirement is less than half of the Calvin cycle, giving bacteria that use the reverse TCA cycle, such as Epsilonproteobacteria, an advantage [7] [8]. Major enzymes in the reverse TCA cycle include ATP citrate lyase, whose role is to split citrate into oxaloacetate and acetyl-CoA [7]. The reverse reactions of the TCA cycle are energetically unfavourable, but these enzymes make the reactions possible [7].
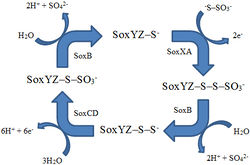
Carbon fixation requires energy, and a main energy source for chemolithoautotrophs at hydrothermal vents is hydrogen sulfide [8]. There are two pathways for sulfur oxidation [8]. The Sox pathway completely oxidizes hydrogen sulfide to sulfate using a multienzyme complex that includes SoxB, SoxCD, SoxXA, and SoxYZ [8]. In the other method, sulfur is first oxidized to a sulfite intermediate and then goes down the adenosine 5’-phosphosulfate pathway, using the enzymes APS reductase and ATP sulfurylase [8].
Key Microorganisms
Epsilonproteobacteria are the dominant class of bacteria of many hydrothermal vents [10]. There are usually both mesophilic and thermophilic species present due to the temperature gradient [10]. One major genus is the chemolithoautotroph Nautilia which is thermophilic and a sulfur-reducer [2]. H2 and formate are the possible electron donors for Nautilia, and sulfur is the electron acceptor, which produces hydrogen sulfide [2]. Another abundant class of bacteria is Gammaproteobacteria, with the chemolithoautotroph Thiomicrospira being the major genus [2]. Thiomicrospira is a sulfide oxidizer, meaning reduced sulfur compounds are used as the electron donors. CO2 is the carbon source [2].
Some hydrothermal vents have fluids that contain lower levels of hydrogen sulfide and carbon dioxide, which are needed by chemolithoautotrophs [12]. A main class of bacteria present at these vents is Deltaproteobacteria, a heterotroph [12]. Methanotrophs are also common at most hydrothermal vents, and shallow sea hydrothermal vents allow for photosynthetic organisms such as cyanobacteria [2].
Current Research
Organisms adapted many of their biomolecules to survive in the hydrothermal vent environment [4]. Examples are the desensitization of cytochrome C oxidase to hydrogen sulfide, and the production of proteins that can bind heavy metals [4]. These modified molecules such as enzymes and nucleic acids may be of use in the fields of medicine and biotechnology [4]. Also of interest are exopolysaccharides that are produced to protect the cell by acting as a layer between the cell and the surrounding environment [1]. Some have been found to have antiviral and immunomodulatory properties [1].
Alkaline hydrothermal vents are hypothesized to potentially be the origin of life on Earth [13]. Arguments for this belief include that at hydrothermal vents, cell-like vesicles form, and nucleic acid building blocks such as ribose, deoxyribose, and nitrogenous bases are made abiotically [13]. Hydrothermal hydrogen is an energy source, and ATP synthesis can be driven by chemiosmosis because of the gradients in temperature, redox, and pH [13]. The existence of sulfur-modified DNA and the 1,6-anhydro bond of peptidoglycan have also been named as molecular fossils that will provide clues to how life originated from these vents [13].
References
(1) Maugeri, T., Bianconi, G., Canganella, F., Danovaro, R., Gugliandolo, C., Italiano, F., Lentini, V., Manini, E. and Nicolaus, B. “Shallow hydrothermal vents in the southern Tyrrhenian Sea.” Chemistry in ecology, 2010, DOI: 10.1080/02757541003693250
(2) Zhang, Y., Zhao, Z., Chen, C., Tang, K., Su, J. and Jiao, N. “Sulfur metabolizing microbes dominate microbial communities in Andesite-hosted shallow-sea hydrothermal systems.” PloS one, 2012, DOI: 10.1371/journal.pone.0044593
(3) Kadar, E., Costa, V., Santos, R. and Powell, J. “Tissue partitioning of micro-essential metals in the vent bivalve Bathymodiolus azoricus and associated organisms (endosymbiont bacteria and a parasite polychaete) from geochemically distinct vents of the Mid-Atlantic Ridge.” Journal of sea research, 2006, DOI: 10.1016/j.seares.2006.01.002
(4) Minic, Z., Serre V. and Herve, G. “Adaptation of organisms to extreme conditions of deep-sea hydrothermal vents.” Comptes rendus biologies, 2006, DOI: 10.1016/j.crvi.2006.02.001
(5) Than, K. “Black smoke rising.” National geographic, 2012, http://news.nationalgeographic.com/news/2012/05/pictures/120523-new-hydrothermal-vents-deep-sea-mexico-mbari-oceans-science/
(6) Van Dover, C. and Lutz, R. “Experimental ecology at deep-sea hydrothermal vents: a perspective.” Journal of experimental marine biology and ecology, 2004, DOI: 10.1016/j.jembe.2003.12.024
(7) Campbell, B. and Cary, S. “Abundance of reverse tricarboxylic acid cycle genes in free-living microorganisms at deep-sea hydrothermal vents.” Applied and environmental microbiology, 2004, DOI: 10.1128/AEM.70.10.6282-6289.2004
(8) Hugler, M., Gartner, A. and Imhoff, J. “Functional genes as markers for sulfur cycling and CO2 fixation in microbial communities of hydrothermal vents of the Logatchev field.” FEMS microbiology ecology, 2010, DOI: 10.1111/j.1574.6941.2010.00919x
(9) Sauve, V., Bruno S., Berks, B. and Hemmings, A. “The SoxYZ complex carries sulfur cycle intermediates on a peptide swinging arm.” J. Biol. Chem., 2007, DOI: 10.1074/jbc.M701602200
(10) Flores, G., Shakya, M., Meneghin, J., Yang, Z., Seewald, J., Geoffwheat, C., Podar, M. and Reysenbach, A. “Inter-field variability in the microbial communities of hydrothermal vent deposits from a back-arc basin.” Geobiology, 2012, DOI: 10.1111/j.1472-4669.2012.00325.x
(11) Perez-Rodriguez, I., Ricci, J., Voordeckers, J., Starovoytov, V. and Vetriani, C. “Nautilia nitratireducens sp. nov., a thermophilic, anaerobic, chemosynthetic, nitrate-ammonifying bacterium isolated from a deep-sea hydrothermal vent.” International journal of systematic and evolutionary microbiology, 2010, DOI: 10.1099/ijs.0.013904-0
(12) Nakagawa, S., Takai, K., Inagaki, F., Chiba, H., Ishibashi, J., Kataoka, S., Hirayama, H., Nunoura, T., Horikoshi, K. and Sako, Y. “Variability in microbial community and venting chemistry in a sediment-hosted backarc hydrothermal system: impacts of subseafloor phase-separation.” FEMS microbiology ecology, 2005, DOI: 10.1016/j.femsec.2005.03.007
(13) Dai, J. “Novel molecular fossils of bacteria: insights into hydrothermal origin of life.” Journal of theoretical biology, 2012, DOI: 10.1016/j.jtbi.2012.06.041
