Influenza Hemagglutinin: Difference between revisions
| Line 18: | Line 18: | ||
===<b>Function</b>=== | ===<b>Function</b>=== | ||
[[Image:3410141_pjab-88-226-g007.png|thumb|500px|right|Conformational changes of influenza HA structure due to pH decrease. Upon acidic conditions, the HA glycoprotein changes from its native to fusion active form, exposing the fusion peptide (green), which is then inserted into the host endosomal membrane, leading to membrane fusion. By Sriwilaijaroen N, Suzuki Y published in 2010]] | [[Image:3410141_pjab-88-226-g007.png|thumb|500px|right|Conformational changes of influenza HA structure due to pH decrease. Upon acidic conditions, the HA glycoprotein changes from its native to fusion active form, exposing the fusion peptide (green), which is then inserted into the host endosomal membrane, leading to membrane fusion. By Sriwilaijaroen N, Suzuki Y published in 2010]] | ||
HA1 and HA2 have different structures and functions. HA1 holds the binding globular head domain and a conserved disulfide bond linking cysteine 52 and cysteine 277, which contribute to its stability [2]. HA1 contains the sialic acid binding site, which binds the virus to the target cell's sialic acid receptor [6]. HA2 consists of the membrane-fusion inducing stalk domain that allows virus particles to enter. The N-terminus of HA2 holds the fusion peptide, which is hidden in the HA structure (green on the right). This region cannot interact with the hydrophobic environment until the pH of the cell decreases, causing the structure to slightly unfold and expose the fusion peptide. This conformational change allows the virus to fuse to the cell, and the virus can now insert its genetic information into the cell. | HA1 and HA2 have different structures and functions. HA1 holds the binding globular head domain and a conserved disulfide bond linking cysteine 52 and cysteine 277, which contribute to its stability [2]. HA1 also contains the sialic acid binding site, which binds the virus to the target cell's sialic acid receptor [6]. HA2 consists of the membrane-fusion inducing stalk domain that allows virus particles to enter. The N-terminus of HA2 holds the fusion peptide, which is hidden in the HA structure (green on the right). This region cannot interact with the hydrophobic environment until the pH of the cell decreases, causing the structure to slightly unfold and expose the fusion peptide. This conformational change allows the virus to fuse to the cell, and the virus can now insert its genetic information into the cell. | ||
The sequence conservation of HA2 (51-80%) is higher than that of HA1 subunit domain (34-59%) [2]. | The sequence conservation of HA2 (51-80%) is higher than that of HA1 subunit domain (34-59%) [2]. | ||
Revision as of 18:40, 13 April 2015
Influenza, also commonly known as the flu, is a contagious respiratory illness caused by various influenza viruses that infect the nose, throat, and lungs [4]. Although influenza is typically a mild and common illness, it has caused 40,000 deaths and 100,000 hospitalizations annually in US. Today, the seasonal influenza vaccine remains the most effective method to prevent the flu.
There are 3 types of viruses: A, B, and C. Type A and type B are the major pathogens in humans, which is why current influenza vaccines are trivalent, consisting of two influenza A subtypes (H1N1 and H3N2) and one or two variants of influenza B virus [2]. Each year, the strains are chosen based on which strains of virus are predicted to most commonly circulate in humans each winter flu season.
Once every year, many receive either "inactivated" or "live, attenuated" flu vaccinations. While recent studies by the CDC show that the seasonal vaccine can reduce the risk of flu illness by approximately 50-60%, these seasonal vaccines only offer short-term and highly specific humoral immunity attributed to the frequent antigenic variations in the influenza virion [1,4]. A solution to these issues is an universal vaccine that offers broad-spectrum immunity. One such conserved antigenic target is the hemagglutinin HA2 stalk domain. If a universal vaccine is successfully made, it will eliminate the need for viral strain forecasting and it will protect against various influenza viruses at once.
Structure, function, and mechanism of hemagglutinin
Structure
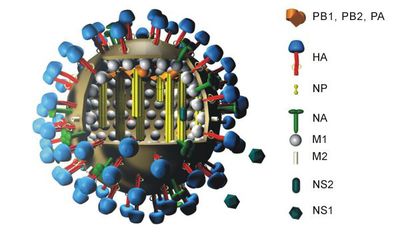
Hemagglutinin (HA) is a glycoprotein on the surface of influenza viruses that causes red blood cells to clump in the presence of an antibody. There are at least 18 HA antigens, and its subtypes are named H1 through H18. They are approximately 13.5nm long and form a cylindrical shape. Hemagglutinin contains three identical monomers from an alpha helix coil, which hold the sialic acid binding sites. Each of these HA monomers has two components: a long, helical chain by HA2 and a large HA1 globule.
Function
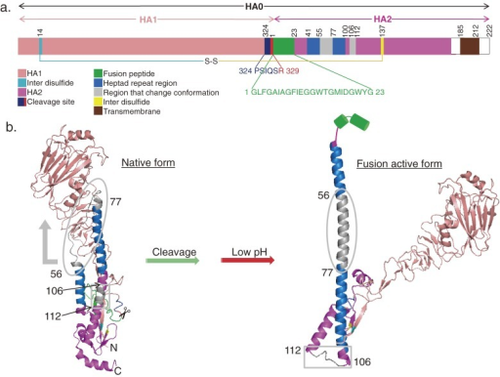
HA1 and HA2 have different structures and functions. HA1 holds the binding globular head domain and a conserved disulfide bond linking cysteine 52 and cysteine 277, which contribute to its stability [2]. HA1 also contains the sialic acid binding site, which binds the virus to the target cell's sialic acid receptor [6]. HA2 consists of the membrane-fusion inducing stalk domain that allows virus particles to enter. The N-terminus of HA2 holds the fusion peptide, which is hidden in the HA structure (green on the right). This region cannot interact with the hydrophobic environment until the pH of the cell decreases, causing the structure to slightly unfold and expose the fusion peptide. This conformational change allows the virus to fuse to the cell, and the virus can now insert its genetic information into the cell.
The sequence conservation of HA2 (51-80%) is higher than that of HA1 subunit domain (34-59%) [2].
Hemagglutinin is the major target of neutralizing antibodies.
Mechanism
Influenza hemagglutinin has two main functions. One function is the HA1 globular head domain binds the virus to the sialic acid receptor on the target site, causing the virus and cell to stick together. A second function is it catalyzes the entry of viral DNA into target cells by membrane fusion [6]. Current influenza vaccines with neutralizing antibodies often replicate these hemagglutinin functions by inhibiting or "neutralizing" attachment of HA to the target cell. Neutralizing antibodies do this by either blocking the sialic acid binding site on HA1 or by inhibiting membrane fusion on HA2. Some propose that a universal influenza vaccine can be promising if...[?]
Hemagglutinin constructs as a universal vaccine
Many current influenza vaccines target the head domain of hemagglutinin. While these vaccines are effective at providing short-term immunity, they have some significant drawbacks [2,3]. One such drawback is the unpredictable mutations surrounding the head domain HA1 that cause these vaccines to have a low broad-spectrum efficacy. The head domain contains variable antigenic sites known as antigenic drift, small changes over time, or antigenic shift, abrupt and large changes. Antigenic drift is caused by frequent point mutations in HA and NA proteins in influenza virions due to lack of proof reading enzymes. The amino acid substitutions in these proteins makes the mutant virions go undetected by host antibodies. Antigenic shift occurs when the fragmented genomes of an influenza virus undergo genetic variations when rare gene exchange occurs between human and non-human (e.g. avian, equine) viral strains, creating a new genotype and novel HA and/or NA proteins.
Due to the frequent and unpredictable mutations caused by antigenic sites, universal influenza vaccines can offer more reliable and broad-spectrum immunity. Many universal influenza vaccine constructs focus on conserved regions of the HA protein [5 steel, Nabel]. HA2 has much less probability of antigenic variation compared to HA1 and it contains a specific region that is conserved among many viral strains [source].
Conserved regions of HA2
One way to construct a vaccine that stimulates an antibody response to a conserved region is by vaccination with headless HA constructs. This novel construct maintains the conserved influenza HA stalk domain (HA2) but lacks the globular head (HA1). The headless HA vaccine capitalizes on the conserved regions in HA in order to offer full protection from death and partial protection against disease following lethal viral challenge in mice [5]. These hemagglutinin constructs have been shown to be safe and successful in preventing infection with influenza A virus in healthy adults and mice [3,5].
Examples of universal vaccine HA constructs
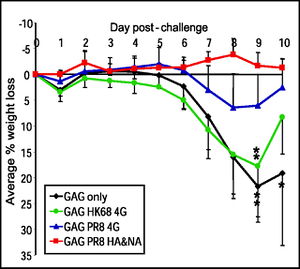
One example of a study that uses a hemagglutinin construct as universal vaccine for influenza of the vaccination of mice with headless HA constructs [5]. Various vaccinations with variations of HA, including vaccinations that lack a globular head domain of HA1 but maintain the integrity of the stalk region, were created and injected into mice twice, on days 0 and 21. On day 77, all of the mice were challenged with a PR8 virus. All of the mice with the headless HA construct survived and showed a small percentage of average body weight loss. In comparison, 3 out of 4 of the mice with the full-length HA died because of the significant body weight loss.
These findings suggest that headless HA constructs are more effective because they attempt to avoid the highly immunogenic head domain. The survival of all the mice with headless HA constructs also shows promising results towards using headless HA as a universal vaccine because it provided full protection from death and partial protection against disease following lethal viral challenge [5]. Headless HA constructs also offer immune sera (make link to Wikipedia) with broader reactivity than full-length HA as well as cross-reactivity against several subtypes of HA.
Conclusion
Current research on various hemagglutinin constructs show that the possibility of a universal vaccine is promising [papers with examples]. By using the conserved region of the stalk domain, an influenza vaccine can be made that greatly eliminates the problem of antigenic variation, which requires a new influenza vaccine with a new strain to be administered each year. More insight to the possibility of a universal cross-protective influenza vaccine can be furthered by new tools of gene-based vaccination and monoclonal antibodies that can be used to conduct more research on the structure-based vaccine design and the development of vaccines that induce an immune response to the highly conserved structural domains shared among many viruses [nature].
Further Reading
References
[6] Stephen Harrison. Influenza Virus Particle. IBiology, 2011. Web. 19 Mar. 2015
Edited by Gabriella Newman, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.