Legume-Rhizobium: Difference between revisions
No edit summary |
No edit summary |
||
| Line 86: | Line 86: | ||
Furseth, B.J., Conley, S.P., and Ane, J-M. 2012. "Soybean Response to Soil Rhizobia and Seed-applied Rhizobia Inoculants in Wisconsin". Crop Science. 52:339-344. | Furseth, B.J., Conley, S.P., and Ane, J-M. 2012. "Soybean Response to Soil Rhizobia and Seed-applied Rhizobia Inoculants in Wisconsin". Crop Science. 52:339-344. | ||
[[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC160880/pdf/070869.pdf Mylona, P., Pawlowski, K. and Bisseling, T., 1995. "Symbiotic Nitrogen Fixation". The Plant Cell. 7:869-885]] | |||
[[http://www.plantmanagementnetwork.org/pub/cm/review/2004/yield/ Iniquez, A. L., Robleto, E. A., Kent, A. D., Triplett, E. W. 2004. "Significant yield increase in Phaseolus vulgaris obtained by inoculation with a trifolitoxin-producing, Hup + strain of Rhizobium leguminosarum bv. phaseoli." Crop Management. March:1-5.]] | [[http://www.plantmanagementnetwork.org/pub/cm/review/2004/yield/ Iniquez, A. L., Robleto, E. A., Kent, A. D., Triplett, E. W. 2004. "Significant yield increase in Phaseolus vulgaris obtained by inoculation with a trifolitoxin-producing, Hup + strain of Rhizobium leguminosarum bv. phaseoli." Crop Management. March:1-5.]] | ||
Revision as of 23:28, 4 April 2012
Introduction
Rhizobia are symbiotic diazotrophs (prokaryotic organisms that carry out dinitrogen fixation) that form a symbiotic association with legumes. This association is symbiotic in that both the plant and rhizobia benefit. The plant supplies the rhizobia with energy in the form of amino acids and the rhizobia fix nitrogen from the atmosphere for plant uptake. The actual process of dinitrogen fixation from the atmosphere can only be carried out by diazotrophs that contain the enzyme dinitrogenase. Nitrogen is the most critical nutrient needed to support plant growth. Unfortunately, atmospheric dinitrogen (78% of air we breathe) is extremely stable due to triple bonds which can only be broken by energy intensive ways. These include electrical N2 fixation by lightning where oxides of N come to ground with rain, the Haber-Bosch process in industrial fertilizer production, and biological N2 fixation in legumes by bacterial symbionts such as rhizobium.

Biological interaction
Biological N2 fixation takes energy which comes at the expense of photosynthate (sucrose). The ATP needed is generated by the electron transport processes occurring at the surface membrane of the bacteroid. 4 ATPs are used per electron in the N2 fixation process. N2 + 6e- + 8H+ 2 molecules NH4+. So a total of 24 ATPs would be required to make 2 molecules of NH4+. Stepwise reaction looks something like this: N2 diamine hydrazine 2NH4+ (Each arrow requires 2 e- for a total of 6 electrons needed) (4 ATP / 1 e- ) x 6 electrons = 24 ATP required.
Communication between legume and rhizobium
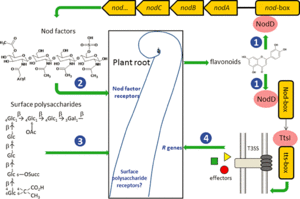
1. Flavonoids are released by the host root. The flavonoid is at the highest concentration at the root and interacts with the product of bacterial nodD gene, leading to the induction of other nodulation genes.
2. Rhizobia colonize the soil in the vicinity of the root hair in response to the flavonoids.
3. The root hair is then stimulated and curls which stimulates cell division in the root cortex.
4. An infection thread is formed as rhizobia digest the root hair cell wall. Free-living rhizobium bacteria are converted to bacteroids.
5. Infection thread branches and a visibly evident nodule develops on the root as the plant produces cytokinin and cells divide.
6. Electrons come in via Fe protein and are donated by ferredoxin.
7. ATP hydrolysis takes place.
8. The Fe protein reduces the MoFe protein which reduces the nitrogen (Dixon and Wheeler, 1986).
9. ATP is used to reduce N2 to 2NH4. The loss of the ammonium assimilatory capacity by bacteriods is important for maintaining the symbiotic relationship with legumes.
Niche
Niche: The amount of N2 fixed depends on the soil population of bacterial symbionts, soil acidity, and often overlooked soil nitrogen availability. Nodulation will only be initiated when the plant is in low nitrogen status. Dintirogenase is unstable in ambient levels of oxygen which causes the enzyme structure to fall apart. Leghemoglobin is responsible for binding oxygen making very little oxygen free giving nodules their pink color.
Environment Suitable
A balanced pH with high levels of nutrients and good physical properties is favored by rhizobia.
Environment Not Suitable
Rhizobia can be reduced in numbers by strong soil acidity, nutrient limitation, poor soil physical properties that restrict aeration and moisture supply, or high soil temperature. Soil acidity reduces nodulation and overall N2 fixation. Furthermore, indegenous rhizobial population depends on how often host plants are grown and on competitive ability of different rhiboia strains (Furseth et al., 2012). Soil nitrate concentration and phosphorus concentration can also affect rhizobia populations.
Microbial processes
Estimates of the amount of N2 fixed range from 57 – 600 kg/ha per year and vary widely. (Evans and Barber, 1977). The rates of N2 fixation vary widely and cannot be measured accurately (LaRue and Patterson, 1982). Legumes prefer to take up soil nitrogen in preference to fixing their own. N2 fixation is constrained in many agricultural soils where nitrogen levels are high from routine addition of fertilizer. Nitrate in the soil reduces fixation where nitrate reduction uses photosynthate. Basically the plant goes the less expensive route.
Key Microorganisms
There are currently six phylogenetically distinct genera of rhizobia.
Allorhizobium
Allorhizobium is a genus that produces nodules on Neptunia prostrata. This plant is an aquatic legume indigenous to humid tropics used for both human consumption and green manure. 1
Azorhizobium
Azorhizobium produce nodules on the aquatic legume Sesbania rostrata. Azorhizobium are unique in that they have the ability to grow with N2 as the only nitrogen source. 1
Bradyrhizobium
Bradyrhizobium differ because the genus grows slowly and is widely known for symbiosis with soybean, but other crops such as peanut, lupine, and cowpea can form symbiosis with Bradyrhizobium. 2
Mesorhizobium
Mesorhizobium produce nodules in trefoils, and several legume species in China. 1
Rhizobium
Rhizobium form symbiosis with vetches, peas, lentil, clovers, and beans.
Sinorhizobium
Sinorhizobium produce nodules in alfalfa, medics, sweetclover, sesbania.
Current Research
Seed-applied Rhizobia Inoculants
Biological fixation of nitrogen can contribute large amounts of plant usable nitrogen to the soil nitrogen pool. Only a small percentage of soybean producers use rhizobia inoculants as a seed treatment in an attempt to maximize the biological fixation of atmospheric nitrogen to plant usable forms in the soil. Results of this experiment indicate that the greatest responses of rhizobia inoculation on yield came on soils having low levels of indigenous rhizobial populations. Yield responded positively to inoculation at only 3 out of 18 environments. The investigators linked low response to high levels of rhizobial populations which were maintained by regular crop rotation with soybean, which kept rhizobial levels sufficient for plant nodulation and rhizobium bacteria reproduction. Researchers concluded that soybean growers should only inoculate soybean seed with rhizobia if soybean had not been grown for 5 or more years at a particular tract of land (Furseth et al., 2012).
Pesticides Reduce Rhizobia Efficiency
Symbiotic nitrogen fixation (SNF) is noted to reduce the need for excessive synthetic fertilizer additions by replacing man-made nitrogen with a naturally produced form. Utilizing crop rotation with legumes could save millions or billions of dollars currently being spent on synthetic nitrogen forms used extensively in monoculture agriculture, namely continuous corn production. Creating environments that will allow chemical signals to be exchanged between the host plant and rhizobia are extremely important in efficient SNF. Phytochemical signals sent out by host plant roots are only specifically recognized by certain soil bacterium leading to high host specificity by rhizobium. Disrupting this signal is costly and was examined by the investigators. The researchers tested five phytochemicals ranging from natural derived phytochemicals to various insecticides (some of which are no longer used in production agriculture, namely DDT) and found that all chemical treatment groups exhibited significantly lower plant yields, inhibited establishment of symbiosis, reduced SNF, and negatively impacted N fixations and plant biomass production (Fox et al., 2007).
Legume-Rhizobium Specificity
References
Evans, H.J., and Barber, L.E., 1977. "Biological nitrogen fixation for food and fiber production. What are some immediately feasible possibilities"? Science. 197:332-339.
Fox, J.E., Gulledge, J., Engelhaupt, E., Burow, M.E., and McLachlan, J.A. 2007. "Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants". PNAS. 104(24):10282-10287.
Furseth, B.J., Conley, S.P., and Ane, J-M. 2012. "Soybean Response to Soil Rhizobia and Seed-applied Rhizobia Inoculants in Wisconsin". Crop Science. 52:339-344.
LaRue, T.A., and Patterson, T.G., 1982. "How much nitrogen do legumes fix"? Adv. Agron. 34:15-38.
Schepers, J.S. and Raun W.R. 2008. Nitrogen in Agricultural Systems. p. 281 - 359. "Russelle, M. P. Biological Dinitrogen Fixation in Agriculture".
Sylvia, D.M., Fuhrmann, J.J., Hartel, P.G., and Zuberer, D.A., 2005. Principles and Applications of Soil Microbiology. 2nd Edition. p. 373 - 404.
Wang, D., Yang, S., Tang, F., Zhu, H., 2012. "Symbiosis specificity in the legume-rhizobial mutualism". Cellular Microbiology: 14(3):334-342.
Young, J.P.W and Johnston, A.W.B. 1989. "The Evolution of Specificity in the Legume-Rhizobium Symbiosis". Tree. 4:341-349
Edited by Blake Meentemeyer, a student of Angela Kent at the University of Illinois at Urbana-Champaign.
