Leptospira Species in the Environment: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
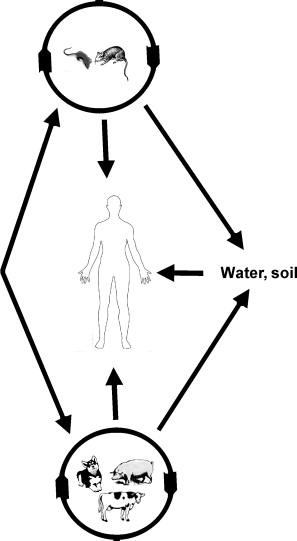
[[Image:Transmissionimage.jpg|thumb|300px|right|Figure 5.Transmission of Leptospirosis between animals and humans.]] | [[Image:Transmissionimage.jpg|thumb|300px|right|Figure 5.Transmission of Leptospirosis between animals and humans.]] | ||
== | ==Genomic Difference Between Pathogenic and Saprophytic Species== | ||
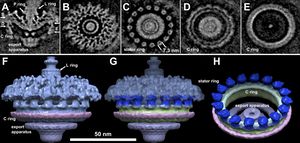
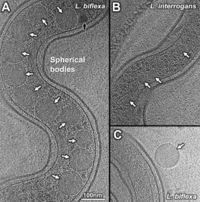
<br> | <br>Leptospira share many of the same features with other spirochetes yet have important differences that allow for the unique shape of the bacteria. Not only do these bacteria differ from other spirochetes, but differ within the genus. The genomic and structural differences within species of the same genus, give rise to the different pathogenic and saprophytic traits of each of the species. Between the pathogenic and saprophytic species, there are around 2,052 common genes that make up the core of the genome. Research has shown that the functional categories of DNA and RNA metabolism as well as protein processing/secretion, and maintenance have been conserved across the varying species, The saprophytic species, L .biflexa, contains a third circular replicon that is absent in the pathogenic species. Most of the genes present in the replicon were for environmental sensing and nutrient acquisition. Because these species rely on the environment and not a host organism, it is imperative that the saprophytic species have these genes to code for the different mechanisms needed to adapt to the various environments. [4,7] Differences can also be seen in the transmission methods of different pathogenic species. For example, L. interrogans can be transmitted from animal to animal/humans though direct contact of infected urine or contaminated water. This transmission method requires the microbe to be in the environment for extended periods of time in between mammalian hosts. On the other hand, L. borgpetersenii cannot survive for long periods of time in the environment and therefore has a transmission method that is much faster than that of L. interrogans. L. borgpetersenii can only be transmitted through direct contact because it is unlikely to survive in the environment. [3]<br> | ||
Revision as of 22:58, 25 April 2014
Introduction
By Toni Miller
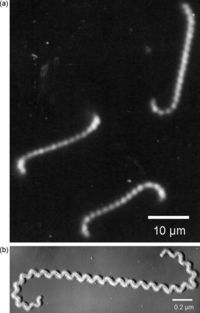
There are various species of Leptospira, all belonging to the spirochete phylum. The Leptospira bacteria are characterized by their unique shape. These Gram-negative bacteria are long and helical in shape, similar to other spirochetes, but contain hooked ends. The hooked ends occur as the result of the two periplasmic flagella located at either pole of the cell. [Fig. 1] These are responsible for the rapid dissemination of the organism into the desired host. [8]Leptospires are obligate aerobes and grow best at temperatures ranging from 28-30°C. [4] One of the most common species of Leptospira is Leptospira interrogans. This species is the most predominant pathogen of the Leptospira phylum and results in a disease called leptospirosis. Leptospirosis is a zoonotic disease predominant in the subtropical and tropical regions of the world. Most cases of leptospirosis in humans originate from a reservoir host, most commonly rodents, and are transmitted through water or contaminated soil. Specifically, L. interrogans colonizes the renal tubules of the host organism. [2] Leptospirosis in livestock can have detrimental effects on the agricultural industry, causing abortions, infertility and death. The effects of leptospirosis infection in humans can ranges from mild symptoms, such as a fever and a headache, to fatal problems causing death. These can include renal failure, jaundice, and pulmonary hemorrhage and are typically termed Weil's disease. [1,4] Contrarily, L. biflexa is a non pathogenic, saprophytic species that is incapable of infecting mammalian hosts. Although sharing many similarities in genetic and structural components, the L. biflexa lacks the genes necessary for virulence. The saprophytic species have been observed to have more genes however, serving as nutrient absorption. [2] With over 13 different species of pathogenic Leptospira and the recorded number of leptospirosis cases well over 1 million annually, Leptospira bacteria are ideal for research. Not only are there vast differences between these spirochetes and others, such as the bacteria responsible for Lyme Disease, but there are also vast differences between each species of Leptospira. [3]

Genomic Difference Between Pathogenic and Saprophytic Species
Leptospira share many of the same features with other spirochetes yet have important differences that allow for the unique shape of the bacteria. Not only do these bacteria differ from other spirochetes, but differ within the genus. The genomic and structural differences within species of the same genus, give rise to the different pathogenic and saprophytic traits of each of the species. Between the pathogenic and saprophytic species, there are around 2,052 common genes that make up the core of the genome. Research has shown that the functional categories of DNA and RNA metabolism as well as protein processing/secretion, and maintenance have been conserved across the varying species, The saprophytic species, L .biflexa, contains a third circular replicon that is absent in the pathogenic species. Most of the genes present in the replicon were for environmental sensing and nutrient acquisition. Because these species rely on the environment and not a host organism, it is imperative that the saprophytic species have these genes to code for the different mechanisms needed to adapt to the various environments. [4,7] Differences can also be seen in the transmission methods of different pathogenic species. For example, L. interrogans can be transmitted from animal to animal/humans though direct contact of infected urine or contaminated water. This transmission method requires the microbe to be in the environment for extended periods of time in between mammalian hosts. On the other hand, L. borgpetersenii cannot survive for long periods of time in the environment and therefore has a transmission method that is much faster than that of L. interrogans. L. borgpetersenii can only be transmitted through direct contact because it is unlikely to survive in the environment. [3]