Membrane proteins as mechanisms of immune system evasion in Trypanosoma brucei
Introduction
Of all defined parasitic human pathogens, the transmitter of Trypanosomiasis (commonly known as African Sleeping Sickness) has a truly fascinating relationship to its hosts. As heteroxenous organisms, Trypanosoma parasites require more than one host to successfully complete their life cycles. Specifically, these flagellate protozoa are unicellular and carry out part of their growth inside the gut of the tse tse fly prior to transmission into the human bloodstream. The mechanisms through which Trypanosoma species evade the human immune system are complex and not completely defined. Much research is concerned with studying the exact modes these unique organisms have for escaping antibody detection and wreaking havoc on their hosts’ health. Perhaps the most important technique Trypanosoma species have is the ability to manipulate their membrane receptor proteins at a more rapid speed than human immune systems can assemble appropriate antibodies. Inadequacy of current treatments has spurred on research to describe this phenomenon in greater detail and discover more effective means of understanding the many facets of African sleeping sickness.
Various species of the genus Trypanosoma confer disease to humans. The most prevalent and thus most widely studied is T. brucei, a eukaryotic flagellate. The parasite is typical in that it contains fully functional mitochondria and other membrane-bound organelles. The mitochondria of the trypanosome include kinetoplasts, disk-shaped networks of condensed circular DNA containing many copies of the mitochondrial genome.
Disease Origins and Distribution
Two morphologically indistinguishable subspecies of Trypanosoma brucei have been described to cause diverse disease patterns in humans: T. b. rhodesiense (East African sleeping sickness) and T. b. gambiense (West African sleeping sickness). The two strains are chiefly found in different regions of Africa and currently no data exists to support any overlap in distribution. Predominantly found in regions of eastern and southeastern Africa, T. b. rhodesiense infections are the most prevalent in Tanzania, Uganda, Malawi, and Zambia (CDC). Wild animals have been found to be the most effective reservoirs of these trypanosomes, capable of infecting local populations as well as sporadically transmitting to hunters and game park visitors. As a result, approximately one case per year is diagnosed in the United States (CDC). While a few hundred incidences of this form of Trypanosomiasis are reported annually, the majority of sleeping sickness cases in African stem from T. b. gambiense, found primarily in central Africa and areas of West Africa (CDC). Trypanosomiasis is most common to the poorest rural populations of some of the least developed Central African countries (Berriman et al 2005). Of the thousands of cases reported to the World Health Organization (WHO) each year, more than 95% originate from Democratic Republic of Congo, Angola, Sudan, Central African Republic, Chad and northern Uganda (Center for Disease Control and Prevention, CDC).
Mechanism of Infection
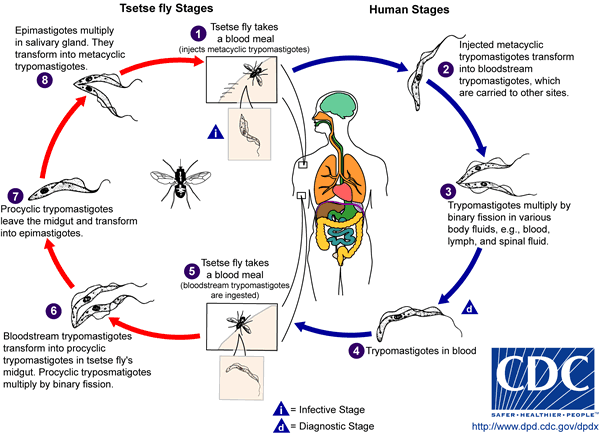
The most common mode of infection is a bite from an infected Glossina palpalis, or tse tse fly (World Health Organization). Tse tse flies occupy mostly rural areas (specifically in woodlands, forests, and vegetation), which is why poorer populations are more exposed to the disease. The fly’s ability to bite and confer the parasite is not inhibited by its sex and it has been reported to only bite during the daylight hours (CDC). The parasite uses the fly as an effective way to gain access to the human blood stream. The infected tse tse fly injects the mammalian host skin tissue with metacyclic trypomastigotes that transform into bloodstream trypomastigotes (Fig. 3). Once inside, T. brucei can multiply freely by binary fission in the blood and lymph. As represented in Figure 3, the trypanosome life cycle in the fly overlaps the parasites infective trajectory in a mammalian host. The tse tse fly ingests bloodstream trypomastigotes from feeding on the blood of infected humans and the parasites transform back into their procyclic forms in the fly midgut. Trypomastigotes multiply and undergo a transformation into epimastigotes, slowly traveling to the fly’s salivary glands where they ready to enter mammalian hosts. This cycle takes around three weeks in the Glossina species (CDC).
While Glossina constitute the vast majority of transmission modes, other mechanisms have been detected. Transmission of the infection from mother to unborn baby has been observed and it could also feasibly be conferred through transfusion of blood or even prolonged blood contact, but such cases are incredibly rare.
Symptoms, Diagnosis, and Treatment
Two subspecies of T. brucei are found to infect humans and each expresses a variation in observable clinical features (CDC). Infection by T. brucei corresponds to initial symptoms of headache, weakness, and even joint pain. If it goes untreated, the disease’s intermediate stages accompany cardiovascular problems, kidney disorders, and anemia (CDC). In its final phase, Trypanosomiasis can lead to extreme exhaustion and fatigue (a feature which gives sleeping sickness its name), coma, and eventually death. The Center for Disease Control and Prevention has described two distinct clinical junctures regarding sleeping sickness. The first is defined by the presence of the parasite solely in the host’s peripheral circulation, not yet in the central nervous system (CNS). Once the blood-brain barrier is crossed and the CNS is infected, the disease is said to have entered its second phase. However, because there are two genetically distinct versions of the parasite, there are also two discrete symptomologies.
The most fundamental distinction between T. b. rhodesiense (East African sleeping sickness) and T. b. gambiense (West African sleeping sickness) is the rate at which their respective infections proceed. The East African variant of the disease progresses rather rapidly, causing a large chancre (or sore) to develop at the site of parasite entry and causing death within months (CDC). T. b. gambiense symptoms appear much more slowly and may be very mild in the beginning, killing the host in no longer than 6-7 years, but more often in about 3 years (CDC). African trypanosomiasis is diagnosed through laboratory methods. Namely, the diagnosis is straightforward and relies on visualization of the parasite in body fluid through microscopy. T. b. rhodesiense parasites are easily observed in blood, whereas T. b. gambiense is easier viewed from microscopic analysis of lymph node aspirate (CDC). Specific drug treatment is directly related to which parasite form a patient has and at what stage the infection is (whether or not the parasite has reached the CNS). Drugs like pentamidine, suramin, melarsoprol, eflornithine, and nifurtimox have been used in the U. S. to combat African trypanosomiasis. Because instances of relapse have been observed, patients are recommended to have serial examinations of cerebrospinal fluid for up to 2 years post treatment (CDC). Sadly, current treatments tend to be inadequate, as drugs intended for late-stage sleeping sickness are highly toxic and no vaccine currently exists to prevent the disease (Berriman et al 2005).
Protein Cycling
African trypanosomes modify their surface membranes in order to evade host immune systems, multiplying with every surface change in a process known as antigenic variation (Berriman et al 2005). The parasite can even elude acquired immunity during infection because of the rate at which they multiply. One of the reasons T. brucei is an incredibly fascinating human pathogen is its ability to undergo rapid membrane protein variation and replicate faster than the immune system can build up a defense against it. A healthy immune system can usually generate antibodies in a relatively brief period, but not fast enough to catch the trypanosomes before their protein compositions are altered. In an effort to better quantify this relationship, the research has been conducted to analyze and sequence the trypanosome genome from its 11 megabase-sized chromosomes (Berriman et al 2005). T. brucei surface membranes have been found to contain invariant antigens that are shielded by an almost impenetrable coat of 107 copies of a single variant surface glycoprotein (VSG) that the parasite replaces as the immune system accumulates antibodies against it (Berriman et al 2005). As of this research, trypanosomes have approximately 806 VSGs (at least that have been characterized thus far), of which most are pseudogenes that the parasites can ectopically recombine to generate expressed mosaic genes. Trypanosomes possess the ability to produce hundreds of different VSG variants, so different in their sequences that host antibodies can’t possibly be effective against all forms (Borst 1982). Additionally, each trypanosome contains genes for each possible VSG, thus it does not have to waste time and expend energy with assemblage like the human immune system does during production of antibodies.
Research is currently being done on the subject of trypanosome membrane protein cycling and its involvement in the pathogen’s ability to successfully infect and avoid immune detection. Work recently published by Koumandou et al (2013) illustrates the important of intracellular trafficking of genes involved in protein recycling with regards to sleeping sickness infection. While much material has been described on the subject of T. brucei’s sophisticated VSG gene-switching mechanism, the data from this report implicate invariant surface glycoproteins (ISGs), as well (Koumandou et al 2013). T. brucei undergoes fairly dramatic membrane protein composition shifts throughout its growth trajectory, utilizing both gene-switching methods and the clearing of antibodies from the cell surface to effectively escape immune detection. Trypanosome gene switching produces VSG proteins that are immunologically distinct from one another and thus the population cannot be entirely eradicated. In addition to these VSG interactions, ISGs have been described to aid in the creation of a therapeutic pathway into the cell (ISGs assist in the uptake of first-line therapeutic agents).
Future Research
further description of mechanism of action (current data)
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
3. Borst, P. "Molecular Basis for Trypanosome Antigenic Variation". Cell. 1982. Volume 29. p. 291-303.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.