Microbial production of recombinant chymosin: Difference between revisions
| Line 59: | Line 59: | ||
Normally, <i>Escherichia coli</i> does not cause disease although some strains frequently cause diarrhea, and it is the most common cause of urinary tract infections. | Normally, <i>Escherichia coli</i> does not cause disease although some strains frequently cause diarrhea, and it is the most common cause of urinary tract infections. | ||
<i>Escherichia coli</i> is one of the most thoroughly studied of all living things. It is a favorite organism for genetic engineering since its genetics and metabolism are the best known. Another advantage of studying <i>Escherichia coli</i> is that it can be grown very easily and inexpensively in a laboratory setting[13,14]. | <i>Escherichia coli</i> is one of the most thoroughly studied of all living things. It is a favorite organism for genetic engineering since its genetics and metabolism are the best known. Another advantage of studying <i>Escherichia coli</i> is that it can be grown very easily and inexpensively in a laboratory setting[13,14]. | ||
Revision as of 04:09, 17 April 2013
Introduction
Cheese production requires the use of a 323 aminoacids protein called chymosin (also known as rennin). Chymosin (36 kDA) is a proteolitic enzyme which is usually obtained from calf stomachs. The role of this enzyme is to coagulate the milk, which is very important for digestion of milk in young animals. Initially, chymosin is secreted as inactive prochymosin and then activated at low pH. Once it is active, it breaks milk protein k-casein in a specific point and effects clotting which is the first step in cheese production. By clotting the milk, a solid product called curd is obtained. Curd is then processed to make cheese[1,2,3].
The increasing world production of cheese, coupled with a decline in the number of slaughtered calves, has stimulated a search for alternative sources of chymosin. One of these alternative sources is the use of recombinant chymosin produced by microorganisms.
Recombinant chymosin is mainly produced by funghi, but it can also be produced using bacteria.
Chymosin mechanism of action
Milk consists of water, fat, protein, phosphate, lactose, citric acid and inorganics such as calcium phosphate. The protein component of milk can be divided into two groups, the casein fraction (αs1-casein, αs2-casein, β-casein and κ-casein)and the whey proteins (β-lactoglobulin, β-lactoalbumin, immunoglobulins and serum albumin). The casein proteins are the ones that will form the curd during cheese making, specifically κ-casein [4].
Chymosin, commonly known as rennin, is the main milk-coagulating enzyme that consists of a single polypeptide chain of 323 amino acids with intramolecular disulfide linkages. Preparations of calf rennet contain two forms of chymosin, A and B. The only difference between chymosins A and B is one amino acid in the polypeptide chain; the chymosin A contains an aspartic acid residue at position 286, whereas the chymosin B contains a glycine residue at the same position [2].
Like other acidic proteases from the gastric juice, chymosin is secreted as an inactive precursor, prochymosin. The precursor is stable at weakly alkaline pH whereas the active enzyme is rapidly denatured at pH values above 7. Below pH 5 prochymosin is converted into chymosin by a limited proteolysis during which a peptide segment is cleaved from the N-terninus. The proteolytic activity of chymosin has optimum pH about pH 3-5.
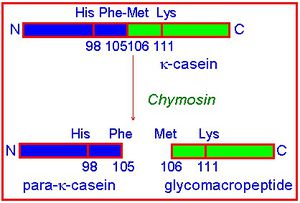
The milk-clotting activity of chymosin is due to proteolysis of the κ-casein. κ-Casein consists of two parts: one hydrophobic and one hydrophilic,in milk, this protein stabilizes the casein micelles against aggregation. During milk-clotting, a Phe-Met bond is hydrolysed, the hydrophilic part of the κ-casein is liberated and aggregation occurs(ref). Chymosin specifically recognises the sequence from His 98 to Lys 111 and cleaves the peptide bond between Phe 105 and Met 106 in the κ-casein chain[5].
Production of recombinant chymosin
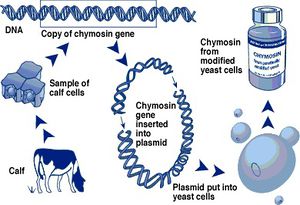
Recombination is the process by which genetic material is broken and then joined to a new genetic material. In this case, the chymosin gene from the cow is taken out of the genome and then introduced into a plasmid. The plasmid is then introduced into a microorganism which will start producing the chymosin by transcribing and translating the gene from the plasmid.
The first step in this process is to obtain a piece of tissue from the calf stomach and isolate the DNA from the cells. After isolating the DNA it is necessary to amplify the chemosyn gene using PCR to increase the amount of gene fragments compared to other sequences of the genome. Another option is to isolate the mRNA directly and turn it into cDNA using the enzyme retrotranscriptase; this way we would have the DNA fragment without the introns as genes are found in prokaryotic microbes.
The second step is to introduce the gene sequence into plasmids that can be then introduced in the target microbes. The gene is often introduced after a promoter sequence that we can control and increase its expression.
After introducing the gene in the plasmid and the plasmid in the microbe, it will start producing chymosin and the last step would be to purify it. The most common way of doing this is using a purification column in which chymosin would stick to the column due to its affinity to the matrix inside the column while the other proteins would not stick[2].
An important fact to be aware of while using recombination of genes from one specie into a different one is the specific variation in codon usage. This problem usually causes low expression levels of the protein that we want to produce. To avoid this problem, the DNA sequence of the gene has to be modified and adapted to the codon usage of the specie in which the chymosin will be produced, this is called codon-optimization[6].
Examples of microbes used in chymosin production
The most common microbes used for the production of recombinant chymosin are funghi, but this protein can also be produced using bacteria. Three of the most used microbes are Aspergillus niger, Kluyveromices lactis and Escherichia coli. These three species has really well known genetics and methabolic pathways, specially Aspergillus niger and Escherichia coli, which make them very useful for recombination experiments like chymosin production.
Aspergillus niger
Aspergillus is the name used for a genus of moulds that reproduce only by asexual means. Aspergillus species are common and widespread. They are among the most successful groups of moulds with important roles in natural ecosystems and the human economy[7]. Aspergillus niger is one of the most common species of this genus[8]. It is found in a large variety of environments, but especially on vegetation, decaying organic matter, and soil. In humans, an Aspergillus niger infection is usually only a problem with people who have a weakened immune system. It most commonly causes lung infections, but it can also spread to other organs and open wounds[9]. Aspergillus niger is really important for industry as it is used also in production of citric acid, gluconic acid and many different enzymes.
Kluyveromyces lactis
Kluyveromyces lactis is a yeast which has the ability to assimilate lactose and convert it into lactic acid[10]. The natural habitat of Kluyveromyces lactis is diverse, but many strains were originally isolated from milk-derived products. It has become an attractive alternative model to Sacharomyces cerevisiae owing to distinct metabolic and physiological properties. Yeasts and fungi are ideal organisms for comparative genomic studies in eukaryotes because of their small and compact genomes[11,12].
Escherichia coli
Escherichia coli is a bacterium that is a common inhabitant of the human colon. It also lives in the intestine of many other animals, wild as well as domestic. It is a gram negative, rod-shaped gammaproteobacteria. It is the model organism for the study of bacteria. Normally, Escherichia coli does not cause disease although some strains frequently cause diarrhea, and it is the most common cause of urinary tract infections. Escherichia coli is one of the most thoroughly studied of all living things. It is a favorite organism for genetic engineering since its genetics and metabolism are the best known. Another advantage of studying Escherichia coli is that it can be grown very easily and inexpensively in a laboratory setting[13,14].
Conclusion
Due to the decrease in the number of slaughtered calves and the ethical problem that killing a huge amount of animals causes, it is important to have an alternative way of producing cheese that does not require the sacrifice of any animal. Moreover, microorganism production of recombinat chymosin offers an easy way of increasing the production of chymosin compared to the amount that can be obtained from young calves. It is important to consider the neccesity of modifying the gene depending on the microbe that is going to be used for production of any recombinant protein due to the variation between species of the codon usage. Taking this into acount may increase dramatically the production of the desired protein.
To sum up, the world usage of microbes to produce any kind of food or useful product is clearly increasing along the years and each day we find that microbes are more and more important in our lifes. Microbes can be bad for us (pathogens) or good (probiotics, industry...) but the fact is that we depend on them much more than we had ever thought.
References
[3] Chymosin (Rennin) and the Coagulation of Milk.
[7] Joan W. Bennett. "An Overview of the Genus Aspergillus".
[10] Eukaryotes Genomes - Kluyveromyces lactis.
[11] [Wésolowski-Louvel, M., K.D. Breunig, and H. Fukuhara, Kluyveromyces lactis. In: K. Wolf, editor. Non-conventional yeast in biotechnology. Heidelberg: Springer- Verlag, 1996: p. 139-201.]
[12] [Lachance, M.-A., Current status of Kluyveromyces systematics. FEMS Yeast Research, 2007. 7(5): p. 642-645.]
[13] E. coli.
[14] Escherichia coli, Streptococcus pyogenes and its treatment.
Edited by Enrique Rodriguez, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.