Naloxone Hydrochloride: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
==Use in EMS and Prehospital Care== | ==Use in EMS and Prehospital Care== | ||
<br> | <br> | ||
[[Image:OpiateGCPR.jpg|thumb|300px| | [[Image:OpiateGCPR.jpg|thumb|300px|middle|Figure 2: Opiate 7-Transmembrane G-Protein Coupled Receptor which when activated inhibits neurotransmitter release. [http://ceaccp.oxfordjournals.org/content/5/1/22.full]] | ||
[[Image:OpiateGCPR.jpg|thumb|300px|left|Figure 2: Opiate 7-Transmembrane G-Protein Coupled Receptor which when activated inhibits neurotransmitter release. [http://ceaccp.oxfordjournals.org/content/5/1/22.full]] | [[Image:OpiateGCPR.jpg|thumb|300px|left|Figure 2: Opiate 7-Transmembrane G-Protein Coupled Receptor which when activated inhibits neurotransmitter release. [http://ceaccp.oxfordjournals.org/content/5/1/22.full]] | ||
Revision as of 03:28, 17 November 2015
Pharmacology of Naloxone Hydrochloride

Naloxone hydrochloride, known by the brand name Narcan, is an opiate antagonist medication used primarily to reverse or lessen the effects of an opiate overdose. It was first synthesized in 1982 by Jack Fishman of Rockefeller University. Later that year, Harold Blumberg of New York Medical College, found that the newly synthesized drug was a “potent, rapid-acting, and relatively pure narcotic antagonist. ”. When delivered into the blood stream it travels to the brain. Naloxone’s hydrophilic properties allows it to easily cross the blood brain barriers where it can make its way to the opiate receptors of the brain and, due to its high affinity for the opiate receptors it outcompetes opiates and binds to the receptors. The three opioid receptors are the mu, kappa and delta receptors. They all have analgesic (pain relieving) properties when activated however the mu receptor also causes respiratory depression. This is important for naloxone because it has the highest affinity for the mu receptor. In a person with any levels of opiates, whether naturally or artificially there, naloxone will prevent those opiates from binding and affecting the opiate receptors. This in turn stops any symptoms of an opiate overdose, and thus the reason for its use in overdoses. Naturally occurring opiates come in the form of endorphins in your body. They are vitally important to short term survival in critical situations. Endorphins will bind to the opioid receptors and create a temporary effect by blocking pain. They are released following injury and can give a person time to remove them selves from a dangerous situation. The effect of endorphins is short lived and they eventually detach from the receptor and pain ensues. This is why you often don’t feel pain immediately after getting injury. When there is no presence of opiates in the brain naloxone will continue to bind to the opiate receptors, however it won’t have any symptomatic effects either positively or negatively. This is due to the fact that it is a pure opioid receptor antagonist. This means that it has no agonist effect, which in this case would be an analgesic effect seen by opiates. Earlier opioid receptor antagonists, like nalorphine, had antagonistic effect on one of the receptors but had an agonist effect on another making it inefficient at treating overdoses. Again for this reason naloxone is an ideal medication for opiate overdoses, because even if it is not an opiate overdose there is no adverse effects of giving the medication. This unique pharmacological effect had lead to the universal adaptation of the use of naloxone in any person with suspected opiate overdose.
Opiate Receptors and Signs of an Overdose
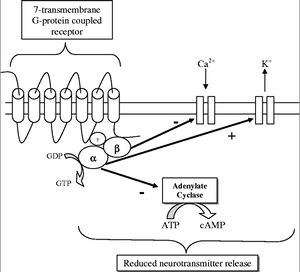
The opiate receptors are 7-Transmembrane G-Protein Coupled Receptors (GPCR), outlined in Figure 2. When the ligand (opiate) binds to the receptor on the transmembrane protein it causes an exchange of GDP for GTP on the alpha subunit of the protein, which causes both the alpha and beta subunit to disassociate from the receptor protein. This disassociation has three effects. The first is the closing of voltage sensitive Ca2+ channels. The second is the pumping out of K+. This hyperpolarizes the neuron. The third step is the inhibition of anenalyte cyclase, which prevents the conversion of ATP to cyclic adenosine monophosphate (cAMP). cAMP is an important secondary cell signaling molecule. All of this contributes to a reduction in function of the cells by not allowing the release of neurotransmitters, which are vital to cell signaling and transmission of a signal. When the GTP is hydrolyzed into GDP the alpha and beta subunits reassemble and rebind to the receptor protein and the ligand disassociates and the signal given by the opiate G-Protein Coupled Receptor is terminated. The inhibition of neurotransmitters in neurons is what gives opiates its analgesic or pain relieving effects. When an excess of opiates are taken there is an effect on breathing due to the lack of the ability of neurons to detect changes in CO2 levels due to dampening of signals passed on by carbon dioxide chemoreceptors. What kills persons from an opiate overdose is respiratory depression leading to respiratory arrest and then cardiac arrest. The signs of current opiate overdose are known as the “opiate overdose triad”. Those three symptoms are pinpoint pupils, unconsciousness, and respiratory depression. Left untreated this will lead to death.
Use in EMS and Prehospital Care


Due to the high prevalence of abuse of opiates, naloxone is especially popular among prehospital EMS responders. Over the counter opiate analgesics account for about 72 percent of the recorded prescription drug abuse cases in the United States. The increasing abuse of illegal opiates such as heroin and opium has also lead to an increasing push for the use of naloxone in prehospital care. Many fire departments carry a similar protocol to the one pictured in Figure 3, taken from the state of New Jersey naloxone drug protocol. As shown the indication for the use of naloxone is “Patients with respiratory depression or arrest secondary to known or suspected opiate overdose (as evidenced by pinpoint pupils, depressed mental status, etc.)”. Due to its very minimal side effects many protocols suggest the use in any sort of “suspected opiate overdose” giving the power to the EMS provider to constitute what that suspicion is. It is for this reason that naloxone has been used almost as diagnostic tool because if the patient does not revert from their symptoms from aggressive treatment of nalxone it is most likely not an opiate overdose and thus the EMS or hospital provider can start trying to figure out another possibility for the current symptoms. This has been furthered by paramedic theory that any unconscious persons of unknown origin should get the administration of D50 (50% dextrose solution used for reversing of diabetic hypoglycemia emergencies) and naloxone since those are the two most likely reasons for a person’s unconsciousness.
There are some varying opinions on the ideal dosage and administration of the drug. The standard naloxone administration set, as shown in Figure 1, comes with a prefilled syringe with 2 milligrams of drug, dissolved in 2 milliliters of saline. This gives it a 1:1 ratio of drug in solution making it easy to administer specific quantities. The quantity given depends greatly on the route of administration. There are three routes used for administration. The first is via a previously established intravenous line (IV line). This is a direct way to administer the drug directly into the bloodstream. As one would expect there is a very quick time of onset associated with this route at around 2 minutes. The downside of this is it requires the establishment of an IV line. This can be difficult due to IV catheterization being a fairly technical skill and the possibility that the veins might not be useable to previous IV drug abuse like heroin. The second route of is via an intra-muscular injection (IM shot). This is just like any normal shot you would get at a doctor. A small gauge hypodermic needle is used to inject the drug into the muscle of the leg or deltoid muscle in the shoulder. As long as perfusion of blood to those muscles is good there is a rapid absorption of it into the blood stream. The issue with IM injections is that during opiate overdoses, especially with respiratory compromise, blood is shunted away from the skin and muscles and to the vital organs in order to keep the person alive. Thus if perfusion of blood to the skin and muscle is poor, then the drug will not be transported to its effector proteins in the brain. Along with that it still requires the use of needles, which requires fairly extensive EMS training to be used. This issue was somewhat solved with the creation of naloxone auto-injectors that work much like an epi-pen. The final method is via intra nasal sprays (IN sprays). This is done by a mucosal atomizer device, also called a mad device. It is attached to the prefilled syringe (as it is included in most naloxone kits) and it creates a spray of the solution, which can be sprayed up the nose where it will absorb into the capillaries of the mucosal membrane. The use of the MAD device has gained popularity due to its easy set up and because it takes little training to be able to be used properly. The downside with this route of administration is that it is the least efficient at delivering the drug to the brain, and thus more of it has to be used.
Dosages as mentioned above varies based on the route of administration. As exemplified by the New Jersey state naloxone protocol (Figure 3), the dosage of naloxone via IN administration is 1 milligram in each nostril resulting in a total dosage of 2 milligrams. The dosage for IM and IV administration is about .4 milligrams due to the more direct route into the blood stream.
Many EMS protocols include the ability to use more of the drug until you reach the desired effect. This is usually written as “titrate to effect”. The “desired effect” is to restore proper respiratory function. As shown by the College Township Fire Department protocol (Figure 4) the dosage can be repeated twice with two to three minute intervals in between. What is often not understood is that a reestablishment of proper respiratory function does not always have to correspond with reestablishing consciousness in the person. Unconsciousness in and of itself is not an immediate danger to a person’s life. The danger comes when you have a threat to airway, breathing or circulation. Ideally naloxone administration would be sufficient to restore proper breathing but not to restore consciousness. This is due to the fact that sudden opiate withdrawal can lead to dangerous side effects for the person as well as the caregiver. Some of the more severe side effects of narcan administration due to sudden opiate withdrawal include nausea, vomiting, diarrhea, and agitation. As experienced by many EMS providers who have successfully used naloxone all four of these can happen to an extreme extent.
Section 3
Include some current research, with at least one figure showing data.
Conclusion
References
Authored for BIOL 291.00 Health Service and Biomedical Analysis, taught by Joan Slonczewski, 2016, Kenyon College.
