Nanotubes facilitate intercellular signaling in eukaryotic and prokaryotic cells: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
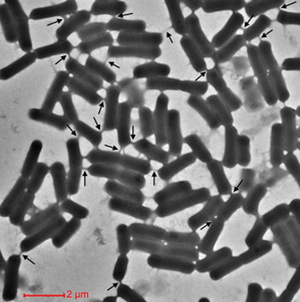
Cells encourage diversity by using several different molecular signaling mechanisms to transfer information, nutrients, organelles, or parasites. Many of these processes require direct cellular interaction for signals to be exchanged. Prokaryotes use conjugation as a means for horizontal gene transfer from one cell to another. Eukaryotes use gap junctions to permit passage of ions and small molecules between the cytoplasm of one cell to another. Many quorum sensing eukaryotes and prokaryotes use extracellular signaling molecules to synchronize their gene expression, behavior, and population density and thus exhibit multicellular properties.[1],[2] Other single-celled or multicellular organisms (and even cancerous cells) release extracellular membrane vesicles or exosomes to shuttle microRNAs, mRNAs, DNA fragments, and proteins to a recipient cell.[3] Additionally, recent research has shown nanotubes (also called tunneling nanotubes (TNTs) or membrane nanotubes (MNTs)) to transiently form between two or more eukaryotic or prokaryotic cells (Figure 1). These nanotubular channels are cell-to-cell plasma membrane connections of varying lengths that allow for bridging cargo exchange and, in some cases, multicellular properties. | |||
Emerging research within the past 10 years has affirmed cellular nanotubes to be involved in complex signaling processes such as viral and prion pathogenesis among eukaryotic cells and metabolic or genetic exchanges among many types of bacteria. Surprisingly, the retrovirus HIV-1 has been shown to move between T-cells, macrophages, and B-cells by use of a nanotube-delivery mechanism for viral proteins.[15],[16],[20],[22] The leukemia-causing HTLV-1 similarly spreads throughout human T-cells by a similar mechanism to HIV-1, and this mechanism allows for a powerful and rapid transmission of the virus throughout the immune system. Prions (PrPSc) that cause degenerative neural disease have been shown to take over nanotubes in bone-marrow derived dendritic cells and spread by them to neural cells, allowing for a safe intercellular route to the brain.[12] Pathogens can indeed utilize already-existing nanotubes between cells, but they have been shown in some cases to even induce nanotube formation.[22] Elucidation of these eukaryotic mechanisms could provide potential targets for drug therapy in otherwise untreatable or hard-to-treat diseases. However, transport of cytoplasmic material through nanotubes is not an activity limited only to eukaryotic cells. Both similar and diverse species of bacteria have been observed to exchange not only cytoplasmic constituents, but also genetic material in the form of plasmids.[19] Dynamic nanotubes have been witnessed between the likes of B. subtilis, E. coli, Staphylococcus aureus, and Acinetobacter baylyi.[18],[19] The new discovery of a stress-induced mutualistic nutrient exchange between distantly related species of bacteria pushes the boundaries of our definition of “unicellular” organisms and prompts us to reevaluate bacteria as a multicellular, interconnected domain of life.[18] | |||
Revision as of 02:47, 21 April 2015
Overview
<http://www.sciencedirect.com/science?_ob=MiamiCaptionURL&_method=retrieve&_eid=1-s2.0-S009286741100016X&_image=1-s2.0-S009286741100016X-figs5.jpg&_cid=272196&_explode=defaultEXP_LIST&_idxType=defaultREF_WORK_INDEX_TYPE&_alpha=defaultALPHA&_ba=&_rdoc=1&_fmt=FULL&_issn=00928674&_pii=S009286741100016X&md5=b2d22cbeb0a19edbc6d02597fb061a4d>.
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Cells encourage diversity by using several different molecular signaling mechanisms to transfer information, nutrients, organelles, or parasites. Many of these processes require direct cellular interaction for signals to be exchanged. Prokaryotes use conjugation as a means for horizontal gene transfer from one cell to another. Eukaryotes use gap junctions to permit passage of ions and small molecules between the cytoplasm of one cell to another. Many quorum sensing eukaryotes and prokaryotes use extracellular signaling molecules to synchronize their gene expression, behavior, and population density and thus exhibit multicellular properties.[1],[2] Other single-celled or multicellular organisms (and even cancerous cells) release extracellular membrane vesicles or exosomes to shuttle microRNAs, mRNAs, DNA fragments, and proteins to a recipient cell.[3] Additionally, recent research has shown nanotubes (also called tunneling nanotubes (TNTs) or membrane nanotubes (MNTs)) to transiently form between two or more eukaryotic or prokaryotic cells (Figure 1). These nanotubular channels are cell-to-cell plasma membrane connections of varying lengths that allow for bridging cargo exchange and, in some cases, multicellular properties.
Emerging research within the past 10 years has affirmed cellular nanotubes to be involved in complex signaling processes such as viral and prion pathogenesis among eukaryotic cells and metabolic or genetic exchanges among many types of bacteria. Surprisingly, the retrovirus HIV-1 has been shown to move between T-cells, macrophages, and B-cells by use of a nanotube-delivery mechanism for viral proteins.[15],[16],[20],[22] The leukemia-causing HTLV-1 similarly spreads throughout human T-cells by a similar mechanism to HIV-1, and this mechanism allows for a powerful and rapid transmission of the virus throughout the immune system. Prions (PrPSc) that cause degenerative neural disease have been shown to take over nanotubes in bone-marrow derived dendritic cells and spread by them to neural cells, allowing for a safe intercellular route to the brain.[12] Pathogens can indeed utilize already-existing nanotubes between cells, but they have been shown in some cases to even induce nanotube formation.[22] Elucidation of these eukaryotic mechanisms could provide potential targets for drug therapy in otherwise untreatable or hard-to-treat diseases. However, transport of cytoplasmic material through nanotubes is not an activity limited only to eukaryotic cells. Both similar and diverse species of bacteria have been observed to exchange not only cytoplasmic constituents, but also genetic material in the form of plasmids.[19] Dynamic nanotubes have been witnessed between the likes of B. subtilis, E. coli, Staphylococcus aureus, and Acinetobacter baylyi.[18],[19] The new discovery of a stress-induced mutualistic nutrient exchange between distantly related species of bacteria pushes the boundaries of our definition of “unicellular” organisms and prompts us to reevaluate bacteria as a multicellular, interconnected domain of life.[18]
Structure
Include some current research in each topic, with at least one figure showing data.
Formation
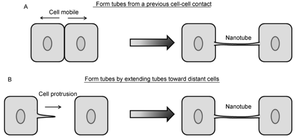
<http://link.springer.com/article/10.1007%2Fs11427-013-4548-3>.
Include some current research in each topic, with at least one figure showing data.
HIV Pathogenesis
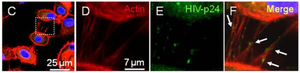
<http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2701345/figure/F5/>.
Include some current research in each topic, with at least one figure showing data.
HTLV Pathogenesis
Overall paper length should be 3,000 words, with at least 3 figures.
Prion Pathogenesis

Include some current research in each topic, with at least one figure showing data.
Prion Pathogenesis
Include some current research in each topic, with at least one figure showing data.
Genetic and cytoplasmic exchange in B. subtilis
Include some current research in each topic, with at least one figure showing data.
Metabolic mutualism between E. coli and Acinetobacter baylyi
Include some current research in each topic, with at least one figure showing data.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
