Nipah Virus: Difference between revisions
Mcquistons (talk | contribs) |
Mcquistons (talk | contribs) |
||
| Line 14: | Line 14: | ||
<br><b>Subscript:</b> H<sub>2</sub>O | <br><b>Subscript:</b> H<sub>2</sub>O | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
Throughout the history of the world humans have been plagued by diseases of various types and origins. Zoonotic diseases, or diseases which have the capability to jump species, animals to humans or vice versa, have been particularly troublesome and deadly. Zoonotic diseases are unique in that they are mainly caused by pathogens such as fungi, bacteria, parasites, or viruses. These pathogens typically survive in a reservoir host, which have immunity to the pathogen. The list of possible reservoir hosts capable of transmitting disease to humans is expansive; however the most common are apes, insects, rodents, and bats. The diseases are then passed to humans who come in contact with an infected animal through bites or scratches, an infected animal’s environment, or animal secretions such as saliva, feces, or mucus. Often these diseases have a higher virulence because of the lack of any immunity within the human population and the ease of transmission. Some more infamous zoonotic diseases are West Nile, Rabies, Ebola, and Dengue fever. | |||
As of recent, more and more zoonotic diseases are emerging because of an increase in human and wildlife interaction. An increase in farming and or deforestation has resulted in humans and wildlife into the same habitat. A prime example of this is the emergence of the Nipah virus (NiV). NiV is a member of the Henipavirus genus in the Paramyxoviridae, and has become a growing concern of Southeast Asia and Australia. | |||
Nipah virus is also a growing concern for the United States. The Center of Disease Control (CDC) has declared it a biosafety level 4 agent (CIDRAP). This the highest biosafety level category, home to agents which can be distributed via aerosol transmission and have no treatment or vaccine. Similar biosafety level 4 agents are Ebola, Smallpox, and several hemorrhagic diseases. The CDC has also tagged the Nipah virus a Category C bioagent, the third highest priority agent category in regards to biological warfare (CIDRAP). The availability, simplicity to produce and disperse, and high mortality rate of the Nipah virus make it possible for it to be used as a weapon of biological warfare. | |||
Revision as of 03:00, 23 April 2013
INTRODUCTION
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Throughout the history of the world humans have been plagued by diseases of various types and origins. Zoonotic diseases, or diseases which have the capability to jump species, animals to humans or vice versa, have been particularly troublesome and deadly. Zoonotic diseases are unique in that they are mainly caused by pathogens such as fungi, bacteria, parasites, or viruses. These pathogens typically survive in a reservoir host, which have immunity to the pathogen. The list of possible reservoir hosts capable of transmitting disease to humans is expansive; however the most common are apes, insects, rodents, and bats. The diseases are then passed to humans who come in contact with an infected animal through bites or scratches, an infected animal’s environment, or animal secretions such as saliva, feces, or mucus. Often these diseases have a higher virulence because of the lack of any immunity within the human population and the ease of transmission. Some more infamous zoonotic diseases are West Nile, Rabies, Ebola, and Dengue fever.
As of recent, more and more zoonotic diseases are emerging because of an increase in human and wildlife interaction. An increase in farming and or deforestation has resulted in humans and wildlife into the same habitat. A prime example of this is the emergence of the Nipah virus (NiV). NiV is a member of the Henipavirus genus in the Paramyxoviridae, and has become a growing concern of Southeast Asia and Australia.
Nipah virus is also a growing concern for the United States. The Center of Disease Control (CDC) has declared it a biosafety level 4 agent (CIDRAP). This the highest biosafety level category, home to agents which can be distributed via aerosol transmission and have no treatment or vaccine. Similar biosafety level 4 agents are Ebola, Smallpox, and several hemorrhagic diseases. The CDC has also tagged the Nipah virus a Category C bioagent, the third highest priority agent category in regards to biological warfare (CIDRAP). The availability, simplicity to produce and disperse, and high mortality rate of the Nipah virus make it possible for it to be used as a weapon of biological warfare.
FUN FACT
The Nipah virus was the virus which inspired the MEV-1 virus in the 2011 Hollywood hit “Contagion”.
MORPHOLOGY OF NiV
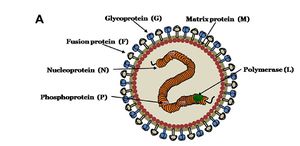
Nipah virus is in the newly created Henipavirus genus with the closely related Hendra virus and Cedar virus. The Henipavirus family is pleomorphic, meaning their shape is varied, and traditionally 40 to 600 nm in diameter. The core of a virion contains a linear ribonucleprotein (RNP) comprising of negative sense single stranded RNA. Also present in the RNP are three critically important proteins. Nucelocapsid proteins (N) are tighly bound to the various nucleotides of the RNA strand. N protein is the most abundant protein present and necessary for capsid structure. Phosphoproteins (P) and large polymerase proteins (L) are also bound to the RNA and aid RNA polymerase in transcribing RNA to mRNA to antigenomic RNA. The virion is enveloped by a traditional lipid bilayer but “spiked” with fusion (F) and receptor-binding glycoproteins (G). The fusion proteins are responsible for fusing the viral membrane to the host membrane triggering the release of the contents of the virion. The receptor-binding glycoporteins are extremely specific and bind only to Ephrin B2 (EFNB2) surface proteins. Specifically, NiV has been found to alternatively bind to EFB3 as well. The EFNB2 surface proteins are highly conserved across the mammalian lineage. On the underside of the lipid bilayer matrix proteins (M) are present for structural support and regulating the budding process. Other proteins, C, V, and W, are also present in the cytoplasm and involved in regulation of transcription and replication.
In regards to the Nipah virus genome, the exact structure is not completely understood. However because of the strong homology between Hendra virus and Nipah virus, a nearly identical structure is hypothesized. The negative sense single stranded RNA is of traditional 3’ to 5’ orientation. All the previously mentioned proteins are encoded by the RNA in the order of 3’-N-P-M-F-G-L- 5’. Similar to all paramyxoviruses NiV RNA replication occurs in the cyptoplasm. All but the P gene are monocystronic, in that they code for a single protein. The P gene also encoded for the C, V, and W proteins which play a role in the virulence of NiV.
Interferons are released by host cells when under attack by a pathogen which enables intercellular communication. The intercellular communication is necessary for the triggering of immune cells which get rid of the pathogen. C, V, and W proteins, encoded by the P gene, have anti-interferon activity in that they block the transcription of interferon signaling. The process by which C, V, and W proteins block the signaling is still unknown.
NiV OUTBREAK HISTORY
The initial outbreak of encephalitis, later discovered to be a result of NiV, and first epicenter occurred in September of 1998 in several pig farms in a small town called Ipoh in northern Malaysia. NiV is thought to have been transmitted through southern Malaysia by the transference of infected pigs to a second epicenter located around Kampung Sungai Nipah. By more transport of infected pigs, as well as human-human transmission, NiV quickly spread into Singapore. This outbreak resulted in 276 infected patients with 106 deaths, a fatality rate of 38%. The main transmission of this outbreak is thought to be from the reservoir host to an intermediate host, to humans. This outbreak also resulted in a huge death rate of the pig population in Malaysia and Singapore.
Since the outbreak in 1998, a dozen outbreaks have since occurred in Bangladesh and India starting in 2001. These outbreaks have resulted in more respiratory disease and a fatality rate of up to 92% which have lead scientists to suspect a different strand of Nipah virus as the culprit. The transmission is thought to be directly from the reservoir host to humans, along with a higher human to human transmission rate.
The most recent outbreak occurred in early February of 2013, resulting in 12 infections and 10 deaths. Overall in Bangladesh, there have now been 188 infections with 146 deaths with a fatality rate of 77%.
OVERVIEW OF NiV TRANSMISSION
As previously stated, zoonotic diseases are spread from animals to humans via a reservoir host. However there can also be an intermediate host, in which the virus amplifies itself. However this intermediate host is not immune to the disease. Through analysis of urine and saliva collected from a suspected reservoir host from the original outbreak in Malaysia, the reservoir host has been identified as the Pteropus fruit bat.
NiV infection has been found in 5 of 14 fruit bat species, with the highest being Pteropus hypomelanus with a 31% infection rate. Despite having a relatively high infection rate, the species of Pteropus fruit bats are not susceptible to the virus. The virus is primarily transmitted via body secretions or partially eaten fruit.
Pigs have been found to be particularly susceptible to NiV as well as highly contagious to each other. Pigs have been identified as an intermediate and an amplifying host. An amplifying host is defined as a host in which the pathogen can become so prevalent a vector, such as a mosquito, can become infectious. Pigs transmit NiV differently than bats in that they shed the virus primarily by coughing and the expulsion of respiratory secretions and saliva. NiV has not been isolated in pig urine. Pigs are primarily infected directly from Pteropus fruit bats or other pigs.
Humans have been found to be infected by three different pathways, Pteropus fruit bat to human, pig to human, and or human to human. Most recent transmission of NiV has been a result of human to human transmission through close contact through respiratory secretions or urine.
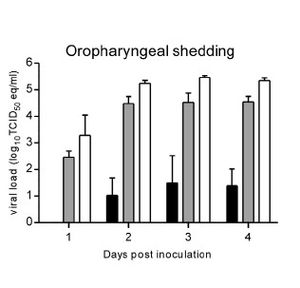
Although not completely understood, the molecular mechanism of Nipah virus transmission is hypothesized to be by fomites. Fomites are non-living objects which are able to carry infectious material like vectors. Currently this is the main hypothesis because of the increased number of NiV infections being picked up in hospitals. It is unknown how long the virus can survive outside a host. Because of the heavy prevalence of virus transmission via respiratory secretions it is assumed the main site of replication occurs in the tonsils of an infected host (CIDRAP). This is supported by research done in the transmission of NiV in hamsters. Wit et al (2011) observed viral shedding of hamsters inoculated with three different doses of varied strength of NiV. The researchers then examined varied forms of shedding of the virus nasally, oropharyngeally, urogenitally, and rectally. The results show, regardless of the dosing, all three dosages resulted in positive viral loads in the oropharyngeal region as soon as two days post inoculation.
The fact that the reservoir and intermediate hosts are Pteropus fruit bats and pigs, respectively, is a primary concern for researchers. Pteropus fruit bats are a migratory species capable of flying long distances. Pigs are also a primary export of Southeast Asia as well as a popular farm animal in the same region. The combination of these two key aspects of the Pteropus fruit bat and pigs makes the transmission of the Nivap virus to other parts of the world extremely easy.
NiV infections have also been identified in cats, dogs, and horses.
MALAYSIA/SINGAPORE OUTBREAK TRANSMISSION OF NiV
BANGLADESH/INDIA OUTBREAK TRANSMISSION OF NiV
REPRODUCTION OF PARAMYXOVIRUSES
PATHOLOGY
DETECTION AND TREATMENT OF NiV
CONCLUSION: THE FUTURE OF NiV
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.