Pathogeny of Borrelia burgdorferi: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
<i>Borrelia burgdorferi </i> is the main bacterium that is responsible for Lyme disease, a tick-borne infectious disease. Lyme disease is one of the most prevalent tick-borne illnesses present in the United States. Ticks infected with <i>Borrelia burgdorferi </i>transmit the bacteria to other organisms. With its painless and almost unnoticeable transmission until after the spread of the bacteria and the bacteria's mechanisms as an pathogenic agent, <i>Borrelia burgdorferi </i> is becoming a widespread public health concern. | |||
==Lyme Disease in the United States== | ==Lyme Disease in the United States== | ||
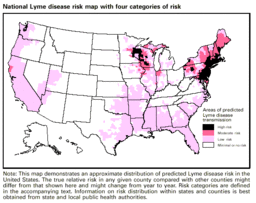
[[Image:Lyme_Disease_Risk_Map.gif |thumb|256px|right|The risk areas of contracting Lyme disease in the United States. By The Center for Disease Control and Prevention (website) [Public domain], via Wikimedia Commons.]] | [[Image:Lyme_Disease_Risk_Map.gif |thumb|256px|right|The risk areas of contracting Lyme disease in the United States. By The Center for Disease Control and Prevention (website) [Public domain], via Wikimedia Commons.]] | ||
<br> There were 19,931 human reported cases of Lyme disease in the United States in 2006 (Shapiro). Reports of Lyme disease are more common in areas that have high populations of ticks. Ixodes, a species of ticks, and in particular Ixodes scapularis (the deer tick), are known to be the predominant couriers of Lyme disease in the United States. Within the United States, these ticks and therefore Lyme disease are generally most found in the Northeastern region, the Northwestern regions, and the Northcentral regions of the United States. Ticks are most active in the warmer seasons of the year (Natural Medicines). Being outdoors, especially in wet and wooded areas increases risk of contact with ticks, as they prefer damp environments. <br> | <br> There were 19,931 human reported cases of Lyme disease in the United States in 2006 (Shapiro). Reports of Lyme disease are more common in areas that have high populations of ticks. Ixodes, a species of ticks, and in particular <i> Ixodes scapularis</i> (the deer tick), are known to be the predominant couriers of Lyme disease in the United States. Within the United States, these ticks and therefore Lyme disease are generally most found in the Northeastern region, the Northwestern regions, and the Northcentral regions of the United States. Ticks are most active in the warmer seasons of the year (Natural Medicines). Being outdoors, especially in wet and wooded areas increases risk of contact with ticks, as they prefer damp environments. <br> | ||
<br> <br> | <br> <br> | ||
| Line 11: | Line 11: | ||
==Morphology== | ==Morphology== | ||
<br> | <br> | ||
[[Image:SpirochaeteSchemamobilityFL.jpg|thumb|256px|right|The morphology of spirochete bacteria and its internal flagella. By Lamiot (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons]]<br>Borrelia burgdorferi are spirochete bacteria, which have flagella that are enclosed between its inner and outer membranes. The flagella of | [[Image:SpirochaeteSchemamobilityFL.jpg|thumb|256px|right|The morphology of spirochete bacteria and its internal flagella. By Lamiot (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons]]<br><i>Borrelia burgdorferi </i> are spirochete bacteria, which have flagella that are enclosed between its inner and outer membranes. The flagella of <i>Borrelia burgdorferi </i>are less subject to environmental factors where the flagella are protected by the double membrane structure. The bacterium’s flagella run lengthwise, rather than branch out into the environment like other bacteria, which allows for <i>Borrelia burgdorferi </i> to better move through harder to maneuver conditions than other bacteria (Tilly). There has been research on the morphology of <i>Borrelia burgdorferi </i> and its role on the bacteria’s success such as <i>Borrelia burgdorferi's </i> ability to transform into cysts in vivo. Research shows that environmental stress can trigger the formation of cysts, making the bacteria more distant and unattainable to outside threats such as the host’s immunological cells. Over 6% of the chromosomal genome of <i>Borrelia burgdorferi </i>i is linked to its mobility (Tilly). The ability of <i>Borrelia burgdorferi </i> to adapt its morphology and motility to the environment proves to be critical for its survival and success. <br> | ||
==Gene Expression/Genome== | ==Gene Expression/Genome== | ||
[[Image: 1471-2180-12-44-2 copy.jpg|thumb|256px|right|A)PCR analysis of ospC transcription before and after tick transmission. B)PCR analysis of ospC transcription in the skin, heart, and bladder in mice at different number of days. By Ouyand et. al, BMC Microbiology 2012 12:44 do:10.1186/1471-2180-12-44. Open Access]] | [[Image: 1471-2180-12-44-2 copy.jpg|thumb|256px|right|A)PCR analysis of ospC transcription before and after tick transmission. B)PCR analysis of ospC transcription in the skin, heart, and bladder in mice at different number of days. By Ouyand et. al, BMC Microbiology 2012 12:44 do:10.1186/1471-2180-12-44. Open Access]] | ||
<br>The genome of | <br>The genome of <i>Borrelia burgdorferi </i> is comparably small to other free-living bacteria, similar to genome sizes of parasites and relies on the host for necessary metabolic machinery (Tilly). Because the actual genome is limited in size, gene expression is extremely important for <i>Borrelia burgdorferi </i>. Because the bacteria’s environments fluctuate from tick hosts to larger animal hosts in regards to temperature and pH, <i>Borrelia burgdorferi </i> is able to adapt to its physiological environment by altering gene expression (Tilly). Research has been conducted to study various genes and the impact of their presence or absence in <i>Borrelia burgdorferi </i>. Two specific genes, the rpoS and rpoN genes, are controlled by signaling cascades that regulate the assembly of various lipoproteins and surface proteins, influencing communication with the environment and driving responses to changing conditions (Tilly). The genome, however, consists of many plasmids that aid the bacterium in gene expression and survival. <i>Borrelia burgdorferi </i> contains ospA, ospB, and ospC plasmid genes that code for major outer-surface proteins, which waver according to the life cycle of the tick. Higher levels of gene expression for the OspC protein are present in the nymphal stage of the tick life cycle than in the larvae and adult stages (Gilmore). Ticks in the nymphal stage are the primary agents of infection, which suggests that OspC might have a role in the bacteria’s pathogeny. <i>Borrelia burgdorferi's </i> proficiency in gene expression allows for its proliferation despite changing environmental factors.<br> | ||
==Host's Immunological Responses== | ==Host's Immunological Responses== | ||
<br>Studies show that host antibodies are able to kill Borrelia burgdorferi in lab settings but not in vivo, suggesting that environmental cues present in host immune systems are essential to their ability to destroy the pathogenic | <br>Studies show that host antibodies are able to kill <i>Borrelia burgdorferi </i> in lab settings but not in vivo, suggesting that environmental cues present in host immune systems are essential to their ability to destroy the pathogenic <i>Borrelia burgdorferi </i>(Embers). The host’s immune system and its responses to the bacteria determine the extent of the bacteria’s pathogenic potency. <i>Borrelia burgdorferi's</i> impact on various organisms such as mice, rabbits, hamsters, and monkeys have been widely studied. Different infectious consequences were seen in the different host organisms. Because of the varying laboratory results, Lyme disease is suggested to be more of a consequence of the host organism’s immunological responses to the bacteria rather than the bacteria’s strategy to be a pathogenic agent (Tilly). Characteristics of <i>Borrelia burgdorferi's</i> such as gene expression of certain surface proteins that influence recognition and attachment of the cell make the bacteria capable of surviving the various levels and means of attack by the host’s immunological cells and molecules, especially in humans. <br> | ||
==Infection in Humans== | ==Infection in Humans== | ||
<br><br> | <br><br> | ||
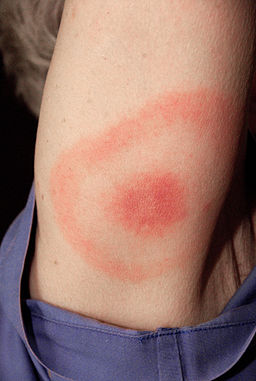
[[Image:skin rash lyme disease.jpg|thumb|256px|right|The skin rash characteristic to the early localized stage of Lyme disease. By James Gathany Content Providers(s): CDC/ James Gathany [Public domain], via Wikimedia Commons]] | [[Image:skin rash lyme disease.jpg|thumb|256px|right|The skin rash characteristic to the early localized stage of Lyme disease. By James Gathany Content Providers(s): CDC/ James Gathany [Public domain], via Wikimedia Commons]] | ||
<br> | <br><i>Borrelia burgdorferi </i>is a slow-growing bacterium, which can result in delayed diagnosis of Lyme disease. Even if Lyme disease is diagnosed early, <i>Borrelia burgdorferi </i> can have the ability to stay dormant for extended period of time and return later in life of the host organism (Natural Medicines). The bacterium attaches to the host organism through the site of a <i>Borrelia burgdorferi </i>-infected tick bite and travels through the bloodstream. At the initial tick bite site, a circular skin rash usually appears within a month and can increase in size over time (Embers). This stage of infection is called the early localized stage. The following stage, the early disseminated stage, is characterized by symptoms of further infection. <i>Borrelia burgdorferi </i> targets various body tissues such as the skin, motor joints, the nervous system, and the cardiac system leading to symptoms like body aches and persistent skin rashes (Natural Medicines). The late stage consists of the same symptoms but with increased intensity, where in some cases can lead to arthritis and deterioration of the nervous system (Embers). <br> | ||
<br><br> | <br><br> | ||
Latest revision as of 06:12, 24 March 2015
Borrelia burgdorferi is the main bacterium that is responsible for Lyme disease, a tick-borne infectious disease. Lyme disease is one of the most prevalent tick-borne illnesses present in the United States. Ticks infected with Borrelia burgdorferi transmit the bacteria to other organisms. With its painless and almost unnoticeable transmission until after the spread of the bacteria and the bacteria's mechanisms as an pathogenic agent, Borrelia burgdorferi is becoming a widespread public health concern.
Lyme Disease in the United States
There were 19,931 human reported cases of Lyme disease in the United States in 2006 (Shapiro). Reports of Lyme disease are more common in areas that have high populations of ticks. Ixodes, a species of ticks, and in particular Ixodes scapularis (the deer tick), are known to be the predominant couriers of Lyme disease in the United States. Within the United States, these ticks and therefore Lyme disease are generally most found in the Northeastern region, the Northwestern regions, and the Northcentral regions of the United States. Ticks are most active in the warmer seasons of the year (Natural Medicines). Being outdoors, especially in wet and wooded areas increases risk of contact with ticks, as they prefer damp environments.
Morphology
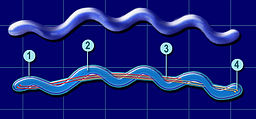
Borrelia burgdorferi are spirochete bacteria, which have flagella that are enclosed between its inner and outer membranes. The flagella of Borrelia burgdorferi are less subject to environmental factors where the flagella are protected by the double membrane structure. The bacterium’s flagella run lengthwise, rather than branch out into the environment like other bacteria, which allows for Borrelia burgdorferi to better move through harder to maneuver conditions than other bacteria (Tilly). There has been research on the morphology of Borrelia burgdorferi and its role on the bacteria’s success such as Borrelia burgdorferi's ability to transform into cysts in vivo. Research shows that environmental stress can trigger the formation of cysts, making the bacteria more distant and unattainable to outside threats such as the host’s immunological cells. Over 6% of the chromosomal genome of Borrelia burgdorferi i is linked to its mobility (Tilly). The ability of Borrelia burgdorferi to adapt its morphology and motility to the environment proves to be critical for its survival and success.
Gene Expression/Genome
The genome of Borrelia burgdorferi is comparably small to other free-living bacteria, similar to genome sizes of parasites and relies on the host for necessary metabolic machinery (Tilly). Because the actual genome is limited in size, gene expression is extremely important for Borrelia burgdorferi . Because the bacteria’s environments fluctuate from tick hosts to larger animal hosts in regards to temperature and pH, Borrelia burgdorferi is able to adapt to its physiological environment by altering gene expression (Tilly). Research has been conducted to study various genes and the impact of their presence or absence in Borrelia burgdorferi . Two specific genes, the rpoS and rpoN genes, are controlled by signaling cascades that regulate the assembly of various lipoproteins and surface proteins, influencing communication with the environment and driving responses to changing conditions (Tilly). The genome, however, consists of many plasmids that aid the bacterium in gene expression and survival. Borrelia burgdorferi contains ospA, ospB, and ospC plasmid genes that code for major outer-surface proteins, which waver according to the life cycle of the tick. Higher levels of gene expression for the OspC protein are present in the nymphal stage of the tick life cycle than in the larvae and adult stages (Gilmore). Ticks in the nymphal stage are the primary agents of infection, which suggests that OspC might have a role in the bacteria’s pathogeny. Borrelia burgdorferi's proficiency in gene expression allows for its proliferation despite changing environmental factors.
Host's Immunological Responses
Studies show that host antibodies are able to kill Borrelia burgdorferi in lab settings but not in vivo, suggesting that environmental cues present in host immune systems are essential to their ability to destroy the pathogenic Borrelia burgdorferi (Embers). The host’s immune system and its responses to the bacteria determine the extent of the bacteria’s pathogenic potency. Borrelia burgdorferi's impact on various organisms such as mice, rabbits, hamsters, and monkeys have been widely studied. Different infectious consequences were seen in the different host organisms. Because of the varying laboratory results, Lyme disease is suggested to be more of a consequence of the host organism’s immunological responses to the bacteria rather than the bacteria’s strategy to be a pathogenic agent (Tilly). Characteristics of Borrelia burgdorferi's such as gene expression of certain surface proteins that influence recognition and attachment of the cell make the bacteria capable of surviving the various levels and means of attack by the host’s immunological cells and molecules, especially in humans.
Infection in Humans
Borrelia burgdorferi is a slow-growing bacterium, which can result in delayed diagnosis of Lyme disease. Even if Lyme disease is diagnosed early, Borrelia burgdorferi can have the ability to stay dormant for extended period of time and return later in life of the host organism (Natural Medicines). The bacterium attaches to the host organism through the site of a Borrelia burgdorferi -infected tick bite and travels through the bloodstream. At the initial tick bite site, a circular skin rash usually appears within a month and can increase in size over time (Embers). This stage of infection is called the early localized stage. The following stage, the early disseminated stage, is characterized by symptoms of further infection. Borrelia burgdorferi targets various body tissues such as the skin, motor joints, the nervous system, and the cardiac system leading to symptoms like body aches and persistent skin rashes (Natural Medicines). The late stage consists of the same symptoms but with increased intensity, where in some cases can lead to arthritis and deterioration of the nervous system (Embers).
Further Reading
Borrelia burgdorferi, the Lyme Disease Agent Journal of Clinical Microbiology, American Society for Microbiology
Lyme Disease Spirochete, Borrelia burgdorferi , Endemic in Epicenter at Turkey Point, Ontario Journal of Medical Entomolgy
References
"Lyme disease Natural Medicines. 2015.
Edited by Jenny Han, a student of Suzanne Kern in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.