Persister Cells in E. coli: Difference between revisions
No edit summary |
|||
| Line 37: | Line 37: | ||
==References== | ==References== | ||
(1) Balaban, Nathalie Q., et al. "A problem of persistence: still more questions than answers?." Nature Reviews Microbiology 11.8 (2013): 587-591. | [(1) Balaban, Nathalie Q., et al. "A problem of persistence: still more questions than answers?." Nature Reviews Microbiology 11.8 (2013): 587-591.] | ||
(2) Balaban, Nathalie Q., et al. "Bacterial persistence as a phenotypic switch." Science 305.5690 (2004): 1622-1625. | [(2) Balaban, Nathalie Q., et al. "Bacterial persistence as a phenotypic switch." Science 305.5690 (2004): 1622-1625.] | ||
(3) Brown, Breann L., et al. "The Escherichia coli toxin MqsR destabilizes the transcriptional repression complex formed between the antitoxin MqsA and the mqsRA operon promoter." Journal of Biological Chemistry 288.2 (2013): 1286-1294. | [(3) Brown, Breann L., et al. "The Escherichia coli toxin MqsR destabilizes the transcriptional repression complex formed between the antitoxin MqsA and the mqsRA operon promoter." Journal of Biological Chemistry 288.2 (2013): 1286-1294.] | ||
(4) Cheow, Wean Sin, Matthew Wook Chang, and Kunn Hadinoto. "Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: the effect of antibiotic release profile." Pharmaceutical research 27.8 (2010): 1597-1609. | [(4) Cheow, Wean Sin, Matthew Wook Chang, and Kunn Hadinoto. "Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: the effect of antibiotic release profile." Pharmaceutical research 27.8 (2010): 1597-1609.] | ||
(5) Dörr, Tobias, Marin Vulić, and Kim Lewis. "Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli." PLoS biology 8.2 (2010): e1000317. | [(5) Dörr, Tobias, Marin Vulić, and Kim Lewis. "Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli." PLoS biology 8.2 (2010): e1000317.] | ||
(6) Fauvart, Maarten, Valerie N. De Groote, and Jan Michiels. "Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies." Journal of medical microbiology 60.6 (2011): 699-709. | [(6) Fauvart, Maarten, Valerie N. De Groote, and Jan Michiels. "Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies." Journal of medical microbiology 60.6 (2011): 699-709.] | ||
(7) Gefen, Orit, and Nathalie Q. Balaban. "The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress." FEMS microbiology reviews 33.4 (2009): 704-717. | [(7) Gefen, Orit, and Nathalie Q. Balaban. "The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress." FEMS microbiology reviews 33.4 (2009): 704-717.] | ||
(8) Gefen, Orit, et al. "Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria." Proceedings of the National Academy of Sciences 105.16 (2008): 6145-6149. | [(8) Gefen, Orit, et al. "Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria." Proceedings of the National Academy of Sciences 105.16 (2008): 6145-6149.] | ||
(9) Hofsteenge, Niels, Erik van Nimwegen, and Olin K. Silander. "Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli." BMC microbiology 13.1 (2013): 25. | [(9) Hofsteenge, Niels, Erik van Nimwegen, and Olin K. Silander. "Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli." BMC microbiology 13.1 (2013): 25.] | ||
<!--Do not remove this line--> | <!--Do not remove this line--> | ||
Revision as of 14:34, 16 March 2014
Bacterial populations form dormant, antibiotic tolerant cells called persister cells. Persister cells are not mutants but phenotypic variants of the wild type 5. Persisters are selected to increase chances of survival in fluctuating environments 2. Persisters are “phenotypic variants” that “enter a non-growing, dormant state.” The dormant state is distinguished by diminished activity of key molecules. Persisters can be distinguished from normal cells due to their reduced growth rate 2. Cells in the persister state are highly immune to bacterial antibiotics, which interfere with processes of key molecules 1. Persisters may represent the long-looked-for explanation for biofilm tolerance to antibiotics 3.
Section 1
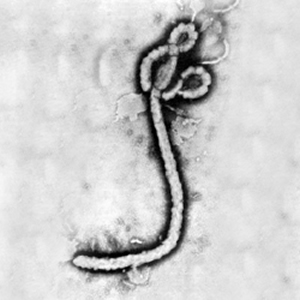
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: Ebola virus 1.jpeg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Overall paper length should be 3,000 words, with at least 3 figures with data.
Section 2
Include some current research in each topic, with at least one figure showing data.
Section 3
Include some current research in each topic, with at least one figure showing data.
Further Reading
[Sample link] Ebola Hemorrhagic Fever—Centers for Disease Control and Prevention, Special Pathogens Branch
References
[(1) Balaban, Nathalie Q., et al. "A problem of persistence: still more questions than answers?." Nature Reviews Microbiology 11.8 (2013): 587-591.] [(2) Balaban, Nathalie Q., et al. "Bacterial persistence as a phenotypic switch." Science 305.5690 (2004): 1622-1625.] [(3) Brown, Breann L., et al. "The Escherichia coli toxin MqsR destabilizes the transcriptional repression complex formed between the antitoxin MqsA and the mqsRA operon promoter." Journal of Biological Chemistry 288.2 (2013): 1286-1294.] [(4) Cheow, Wean Sin, Matthew Wook Chang, and Kunn Hadinoto. "Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: the effect of antibiotic release profile." Pharmaceutical research 27.8 (2010): 1597-1609.] [(5) Dörr, Tobias, Marin Vulić, and Kim Lewis. "Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli." PLoS biology 8.2 (2010): e1000317.] [(6) Fauvart, Maarten, Valerie N. De Groote, and Jan Michiels. "Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies." Journal of medical microbiology 60.6 (2011): 699-709.] [(7) Gefen, Orit, and Nathalie Q. Balaban. "The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress." FEMS microbiology reviews 33.4 (2009): 704-717.] [(8) Gefen, Orit, et al. "Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria." Proceedings of the National Academy of Sciences 105.16 (2008): 6145-6149.] [(9) Hofsteenge, Niels, Erik van Nimwegen, and Olin K. Silander. "Quantitative analysis of persister fractions suggests different mechanisms of formation among environmental isolates of E. coli." BMC microbiology 13.1 (2013): 25.]
Edited by (your name here), a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.