Phage Therapy: Difference between revisions
| Line 19: | Line 19: | ||
<br> It is clear that before phage therapy can be used clinically there must be many more studies that can pinpoint more fully how to best make and administer phages. One potential problem is that just as bacteria are able to become resistant to antibiotics, they may also be able to develop resistances to phages (Inal 2003). There may be development of antibodies if phages are administered multiple times. This is easy to overcome by using instead of one type of phage, a cocktail of different ones. Another more significant potential issue is the possibility that the spleen will take up and deactivate the phages before they have the opportunity to reach their targets. This again reflects the need for more in vivo studies that can elucidate how phages actually react the the body and vice versa (Inal 2003). | <br> It is clear that before phage therapy can be used clinically there must be many more studies that can pinpoint more fully how to best make and administer phages. One potential problem is that just as bacteria are able to become resistant to antibiotics, they may also be able to develop resistances to phages (Inal 2003). There may be development of antibodies if phages are administered multiple times. This is easy to overcome by using instead of one type of phage, a cocktail of different ones. Another more significant potential issue is the possibility that the spleen will take up and deactivate the phages before they have the opportunity to reach their targets. This again reflects the need for more in vivo studies that can elucidate how phages actually react the the body and vice versa (Inal 2003). | ||
==Delivery to targeted bacteria== | ==Application== | ||
===Delivery to targeted bacteria=== | |||
<br> When phages were first discovered and applied in a clinical setting for the treatment of diseases, they were injected in the vicinity of the infection. Later mechanisms of delivery included topical administration 3 times a day, eye drops, or orally before meals (Inal 2003). Phages, unlike many antibiotic molecules, are not diffusible across membranes and must therefore have a method of delivery to the target cells. Some researchers believe that the best delivery mechanism may lie in using other nonpathogenic species of bacteria to bring the phage to its pathogenic target. | <br> When phages were first discovered and applied in a clinical setting for the treatment of diseases, they were injected in the vicinity of the infection. Later mechanisms of delivery included topical administration 3 times a day, eye drops, or orally before meals (Inal 2003). Phages, unlike many antibiotic molecules, are not diffusible across membranes and must therefore have a method of delivery to the target cells. Some researchers believe that the best delivery mechanism may lie in using other nonpathogenic species of bacteria to bring the phage to its pathogenic target. | ||
| Line 28: | Line 29: | ||
<br> Some researchers have categorized different delivery modes as 'passive' and 'active'. Passive delivery is achieved by administering large doses of phage that likely exceed how many bacteria are present. Active delivery, on the other hand, involves making sure that the phage lives long enough to generate progeny cells which will then kill the bacterial cells. This latter category has the advantage that it can act over a longer period, but it may be difficult to realize fully because new doses must be administered at key time intervals. A study by Platt et al (2003) tried to employ a lysogenic life cycle such that cells would burst periodically and release phage into the environment. They used a non-pathogenic strain of <i>E. coli</i> that was lysogenic for a lytic mutant. They suggest that this is the best way to constantly release phage into the environment. They also say that this system may get around the issue of phage-resistant mutants by including different lysogens for different receptors on the target pathogen (Platt et al 2003). | <br> Some researchers have categorized different delivery modes as 'passive' and 'active'. Passive delivery is achieved by administering large doses of phage that likely exceed how many bacteria are present. Active delivery, on the other hand, involves making sure that the phage lives long enough to generate progeny cells which will then kill the bacterial cells. This latter category has the advantage that it can act over a longer period, but it may be difficult to realize fully because new doses must be administered at key time intervals. A study by Platt et al (2003) tried to employ a lysogenic life cycle such that cells would burst periodically and release phage into the environment. They used a non-pathogenic strain of <i>E. coli</i> that was lysogenic for a lytic mutant. They suggest that this is the best way to constantly release phage into the environment. They also say that this system may get around the issue of phage-resistant mutants by including different lysogens for different receptors on the target pathogen (Platt et al 2003). | ||
===Effectiveness=== | |||
<br> Many of the recent studies that have looked at the effectiveness of phage therapy (in vitro and in vivo) have found that it can be very helpful in killing bacteria. One study by Huff et al (2003) found that treating chickens suffering from severe respiratory infections cause by <i>E. coli</i> was very helpful in clearing up symptoms. They found that the application of bacteriophage was most useful very soon after the chickens had been exposed to the bacteria and that, if treated early, multiple doses are better than a single dose. Interestingly, if treatment starts later, there is no difference between single or multiple doses, but treatment is still very helpful. Overall, this study found that phage therapy does seem to have a promising future and is very effective as an alternative to antibiotics. However, it is important to point out that phage therapy is the same as antibiotics in the necessity to catch the disease early (Huff et al 2003). | |||
==Comparison to antibiotics== | ==Comparison to antibiotics== | ||
Revision as of 22:39, 18 April 2010
Introduction
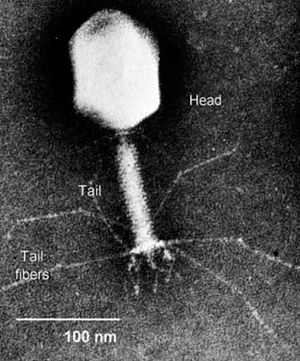
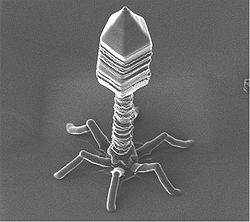
Bacteriophages are viruses that infect bacteria. Bacteriophages have many potential applications in biotechnology that are currently being explored such as being used as delivery vehicles for vaccines and gene therapy, detecting bacterial pathogens, and as a way to screen libraries of peptides or antibodies. Phage therapy takes advantage of this particular group of viruses in a relatively simple way; it aims to treat bacterial infections through the application of such phages.
Though for some time people did not consider this use of bacteriophages feasible, since the emergence of many antibiotic-resistant pathogens, then field is again on the rise (Inal 2003). Recent WHO figures suggest that 14,000 people die in the US alone every year due to multidrug-resistant bacteria acquired in hospitals. Luckily, bacteriophages are found in all bacteria so the is at least a theoretical possibility that therapies can be developed for all species of bacteria (Inal 2003). Because humans are unlikely to be able to come up with novel antibiotics forever and bacteria will probably continue to evolve resistances, from the human perspective it is important that new ways of thwarting infection are discovered.
It seems that bacteriophages may be the knight in shining armor for the treatment of bacterial infections. They are specific to their bacterial host and therefore do not harm humans or plants, they can grow in numbers as long as the bacteria are present, and they are self-limiting in that once they kill all of the target bacteria they no longer have a place to live and they die off too (Summers 2001; Clark and March 2006).
History
Early research
Felix d'Herelle is usually credited with the first cogent description of bacteriophages (Summers 2001). He was studying dysentery and described certain "invisible microbes" that he found in some of his patients without bacteria in their stool, and he correctly attributed this to the action of the virus as some kind of recovery agent. Phage therapy was first attempted soon after the discovery of bacteriophages as d'Herelle tested out their therapeutic effectiveness on first animals (chickens and cows, to good success) and then humans. Later, a group in India began studying the usefulness of bacteriophages in the treatment of cholera and found that symptoms were reduced in severity and length when the patient was treated with phage, which is indicative of many other positive results from treatment with bacteriophages that were also seen around that time (Summers 2001). William Summers has divided the history of phage therapy into four periods: early enthusiasm, critical skepticism, abandonment, and recent interest and reappraisal. Though there was a fair amount of positive findings on the effectiveness of phage therapy, there were also some negative findings. As research interest waned in the West due to World War II, the rise in the use of antibiotics, and some early inconsistent and unsuccessful results, phage therapy was still being used on the battlefront by both the Russians and the Germans. For economic and cultural reasons research on phage therapy grew and expanded in the Soviet Union while the West separated itself and spent more time and money on discovering novel antibiotics. Therefore the countries with the richest history of phage research are Russia and Georgia (Summers 2001).
Early issues
There are several reasons why the early trials in bacteriophage therapy were unsuccessful (Weld et al 2004). For one, phage biology was poorly understood, and therefore the wrong phage or the wrong concentration of phage may have been given to treat an infection. Another limiting factor to the success of early therapeutic uses of phage is that the preparations of phage to be administered were all too commonly contaminated or did not contain enough viable viruses to be effective (Weld et al 2004). The biology of the bacteriophage was not well-understood and so many of the tests that showed inconsistent effectiveness of phage therapy was due to the fact that what was administered was not what was needed to do the job. By the time the underlying biology of bacteriophages was better understood, interest had waned and there were few who cared to go back and make new experimental treatments with the new information ans a foundation (Summers 2001). Lastly, early attempts were not consistently effective because sometimes the wrong phage would be employed or they would choose phage-resistant bacteria to study. Current thinking to avoid this issue involves using cocktails of slightly different bacteriophages to target all of the pathogenic bacteria, even those with mutations or plasmids protecting them from certain phage. Though it is less likely that these same mistakes will be encountered in current use of bacteriophages in a therapeutic setting, there still need to be significant research programs undertaken before phage therapy will be fit for widespread use (Weld et al 2004).
Current research and potential problems
Though current research appears promising and it seems that phage therapy may be a good solution to the problem of multidrug resistant bacteria, there is a long way to go before the therapy is ready to be applied on a widespread basis. One current issue is that many of the experiments that are done are in vitro, meaning that the same things that are observed may not be seen when ones goes to a larger and more complicated environment (Weld et al 2004). New growth models are needed that can be extrapolated to in vivo growth. Weld et al (2004) attempted to make new models for phage growth, but quickly found that bacteriophages have a far more complicated growth cycle in vivo. They found not only that it was difficult to extrapolate from in vitro to in vivo, but also that it was hard to make generalizations about all in vivo situations from observing one (Weld et al 2004).
It is clear that before phage therapy can be used clinically there must be many more studies that can pinpoint more fully how to best make and administer phages. One potential problem is that just as bacteria are able to become resistant to antibiotics, they may also be able to develop resistances to phages (Inal 2003). There may be development of antibodies if phages are administered multiple times. This is easy to overcome by using instead of one type of phage, a cocktail of different ones. Another more significant potential issue is the possibility that the spleen will take up and deactivate the phages before they have the opportunity to reach their targets. This again reflects the need for more in vivo studies that can elucidate how phages actually react the the body and vice versa (Inal 2003).
Application
Delivery to targeted bacteria
When phages were first discovered and applied in a clinical setting for the treatment of diseases, they were injected in the vicinity of the infection. Later mechanisms of delivery included topical administration 3 times a day, eye drops, or orally before meals (Inal 2003). Phages, unlike many antibiotic molecules, are not diffusible across membranes and must therefore have a method of delivery to the target cells. Some researchers believe that the best delivery mechanism may lie in using other nonpathogenic species of bacteria to bring the phage to its pathogenic target.
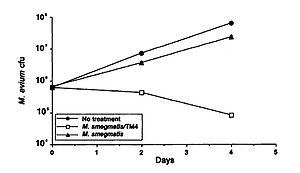
A study by Broxmeyer et al (2002) investigated the effectiveness of phage treatment of an intracellular human pathogen by using a mouse model. The study tested the ability of a mycobacteriophage delivered by a nonvirulent mycobacterium to kill Mycobacterium avium and Mycobacterium tuberculosis. These disease are common in sufferers of acquired immunodeficiency syndrome (AIDS), and though there have been some advancements in treatment in recent years, their effectiveness is limited by drug resistance and the fact that most of their modes of action require that the target is not in a dormant period. The researchers used vacuoles within macrophages to deliver the phage and found that treatment of M. avium-infected and to an even greater extent M. tuberculosis- infected cells with the phage resulted in decreased bacterial numbers (Figure 1). They capitalized on the fact that infecting macrophages with both M. avium and Mycobacterium smegmatis (the delivery bacteria for the TM4 lytic phage) leads to fusion of the vacuoles of the two, which in turn results in delivery of the phage to the target pathogenic bacteria. A decrease in M. avium number was seen when treated with the delivery bacteria, but a far greater decrease was seen when it was coinfected with the delivery bacteria plus the TM4 phage (Figure 1). (Broxmeyer et al 2002).
This study provides promising support to the potential for the use of other bacteria as a delivery mechanism for bacteriophages to treat pathogenic diseases.
Some researchers have categorized different delivery modes as 'passive' and 'active'. Passive delivery is achieved by administering large doses of phage that likely exceed how many bacteria are present. Active delivery, on the other hand, involves making sure that the phage lives long enough to generate progeny cells which will then kill the bacterial cells. This latter category has the advantage that it can act over a longer period, but it may be difficult to realize fully because new doses must be administered at key time intervals. A study by Platt et al (2003) tried to employ a lysogenic life cycle such that cells would burst periodically and release phage into the environment. They used a non-pathogenic strain of E. coli that was lysogenic for a lytic mutant. They suggest that this is the best way to constantly release phage into the environment. They also say that this system may get around the issue of phage-resistant mutants by including different lysogens for different receptors on the target pathogen (Platt et al 2003).
Effectiveness
Many of the recent studies that have looked at the effectiveness of phage therapy (in vitro and in vivo) have found that it can be very helpful in killing bacteria. One study by Huff et al (2003) found that treating chickens suffering from severe respiratory infections cause by E. coli was very helpful in clearing up symptoms. They found that the application of bacteriophage was most useful very soon after the chickens had been exposed to the bacteria and that, if treated early, multiple doses are better than a single dose. Interestingly, if treatment starts later, there is no difference between single or multiple doses, but treatment is still very helpful. Overall, this study found that phage therapy does seem to have a promising future and is very effective as an alternative to antibiotics. However, it is important to point out that phage therapy is the same as antibiotics in the necessity to catch the disease early (Huff et al 2003).
Comparison to antibiotics
Though phage therapy aims to do the same thing as antibiotics in treating bacterial infections, they have different mechanisms and therefore different associated advantages and disadvantages.
Advantages
One advantage of phage therapy is that phages cannot grow without their target bacteria. Therefore, once they have fulfilled their purpose and killed the pathogen, they too will die. Another advantage is they would be easy to administer orally, intravenously, or topically. Phages are also more host-specific than antibiotics, so it is less likely that there will be "collateral damage" relating to killing normal and healthy gut flora along with the pathogen (Clark and March 2006). This high specificity is also important because it reduces the potential for secondary infections to develop (Inal 2003). If administered intravenously, it is easy for the bacteriophages to spread rapidly throughout the body and they can also multiply on their own in vivo, which is important because repeated doses are therefore unlikely to be needed. This proliferation of progeny in vivo would also help in topical applications because they would be more able than antibiotics to infiltrate wounds and reach more of the bacteria (Clark and March 2006).
Antibiotics often have a problem reaching bacterial targets, especially if those targets have formed a biofilm that the antibiotics cannot penetrate. Some bacteriophages synthesize enzymes that allow the breakdown of these biofilms so that the interior cells can be reached and lysed (Clark and March 2006). Bacterial resistance to phages is also less of a concern than resistance to antibiotics (Inal 2003). Even if resistance develops to a bacteriophage, finding a phage that will still work against that bacteria would only take a couple of days. Phages mutate just as much or more than bacteria, so they can better keep up with the bacteria evolving resistances. This ability to continue to grow and multiply in the body is another advantage of phage therapy because while antibiotics are broken down by the body and then excreted, bacteriophages are able to continue to keep their populations up and even grow in number as long as the target bacteria is present. Another major advantage is that bacteriophages are cheap to produce and the years of experimentation in Russia and Georgia have shown almost no side effects at all (Inal 2003).
Disadvantages
There are also some disadvantages to phage therapy. For example, though they would be easy to administer orally, this may lead to a need to neutralize the stomach acid before ingesting in order to minimize damage to the phage or to the delivery bacteria, which may not be able to survive at such a low pH. For the intravenous application of bacteriophages, they may be cleared by the human immune system which would recognize tham as foreign and a possible threat. The phage would also need to be highly purified so that there is no dangerous contaminant or toxin going into the patient's circulatory system. Topical application of bacteriophages to treat infection may also have disadvantages again because of interference by the immune system and topical treatment may also require continuous treatment (Clark and March, 2006). The fact that bacteriophages are so strongly specified for their bacterial target, though in some ways an advantage, can also serve as a disadvantage (Inal 2003). This is because the exact bacteria must be diagnosed in order to use the right kind of phage. Antibiotics, on the other hand, affect a wider range of bacteria, so an exact diagnosis is not essential (Inal 2003).
A more non-scientific downside to the use of phage therapy is that due to intellectual property laws and the fact that currently its use is public, the technique of phage therapy would not be patentable. The development of drugs is very expensive and larger pharmaceutical companies have no motivation to go through the complicated and costly process is there is no patent protection on the products they may create.
The future of phage therapy
Possible novel uses
Bacteriophages can be used in many applications related to therapy besides pathogens alone (Inal 2003). For example, it may be possible to use the products of phages rather than the whole viral organism. Besides simplifying the questions related to whether the bacteriophage could survive in the complex in vivo environment of the human body, this would also be very specific to the bacterial hosts. If peptides such as lysins could be isolated from phages that do things like break down the peptidoglycan in the bacterial cell wall, then it may be possible to administer the protein alone. This would be good because many of these peptides are specific up the the subspecies level, and it has been found that the mutations that the bacteria would require to resist this lysis would also kill the bacteria (Inal 2003).
Another possible use of bacteriophages is against bacteria in agriculture and fisheries (Inal 2003). Early on, a likely application of phage therapy will be to control the outbreaks of bacterial infections in crops. Another application may be in fisheries to limit infections of the fish, crustaceans, etc. In fact, the control of fish pathogens through phage therapy is already in practice today and has been very successful. This is probably due to the fact that the liquid environments that they are placed in to be close to the fish are very close to the liquid environments where they were found. This skirts much of the issue relating to the in vivo difficulty of environment and mediates contact between the phage and the host (Inal 2003).
Obstacles
One obstacle to the widespread use of phage therapy is the public view of viruses. Since they have such a negative connotation in many cultures, it would take some education to cross that "psychological barrier" (Inal 2003). However, while there may initially be qualms about using a virus, it would probably be easy to move past once it was explained how many things actually use live viruses. For example, many of the routine vaccines given to people consist of live viruses. Additionally, viruses are everywhere, and most importantly, not all of them (indeed, most of them), will not cause people harm (Inal 2003).
Conclusion
Bacterial resistance to antibiotics is a growing threat in our world. Though early studies of phage therapy did not produce consistently favorable results, there is reason to believe that with the enhanced understanding that we have of viral biology it is likely that bacteriophages can be a helpful therapeutic tool. Though many believe that phages will not replace antibiotics right away or maybe ever, there is definite potential for their use in conjunction with antibiotics (Clark and March 2006).
References
Broxmeyer, L., Sosnowska, D., Miltner, E., Chacon, O., Wagner, D., McGarvey, J., Barletta, R.G., and Bermudez, L.E. "Killing of Mycobacterium avium and Mycobacterium tuberculosis by a Mycobacteriophage Delivered by a Nonvirulent Mycobacterium: A Model for Phage Therapy of Intracellular Bacterial Pathogens". The Journal of Infectious Diseases. 2002. Volume 186, Number 8. p. 1155-1160.
Clark, J.R. and March, J.B. "Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials". TRENDS in Biotechnology. 2006. Volume 24, Number 5. p. 212-218.
Inal, J.M. "Phage Therapy: a Reappraisal of Bacteriophages as Antibiotics". Archivum Immunologiae et Therapiae Experimentalis. 2003. Volume 51. p. 237-244.
Platt, R., Reynolds, D.L., and Phillips, G.J. "Development of a novel method of lytic phage delivery by use of a bacteriophage P22 site-specific recombination system". FEMS Microbiology Letter. 2003. Volume 223. p. 259-265.
Summers, W.C. "Bacteriophage Therapy". Annual Review of Microbiology. 2001. Volume 55. p. 437-451.
Weld, R.J., Butts, C., and Heinemann, J.A. "Models of phage growth and their applicability to phage therapy". Journal of Theoretical Biology. 2004. Volume 227. p. 1-11.