Phyllosphere: Difference between revisions
| Line 31: | Line 31: | ||
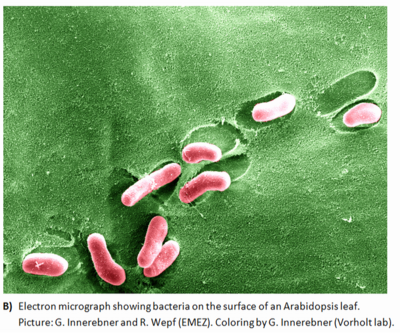
to see a photo of phyllospheric bacteria click the link [microbial interactions] | to see a photo of phyllospheric bacteria click the link [microbial interactions] | ||
Given that water availability is likely one of the most highly fluctuating factors on leaf surfaces, the heavy EPS slime within aggregates may shield the bacteria from desiccation stress by buffering the matric and osmotic potentials of their surroundings. Additionally, EPS has a role in protecting plant-associated bacteria from reactive oxygen species, which are often encountered on plants. It has been demonstrated that aggregated bacteria resist oxidative stress better than planktonic bacteria (1,7). | Given that water availability is likely one of the most highly fluctuating factors on leaf surfaces, the heavy EPS slime within aggregates may shield the bacteria from desiccation stress by buffering the matric and osmotic potentials of their surroundings. Additionally, EPS has a role in protecting plant-associated bacteria from reactive oxygen species, which are often encountered on plants. It has been demonstrated that aggregated bacteria resist oxidative stress better than planktonic bacteria (1,7). | ||
Revision as of 08:27, 22 April 2010
Introduction
The [phyllosphere]refers to the above-ground surfaces of a plant as a habitat for microorganisms, with a heavy emphasis on leaf surfaces. All plants are home to a wide variety of microorganism communities including bacteria, fungi, and yeasts. Some microorganisms benefit the plant, others are plant pathogens and can potentially damage or kill the host plant (4). The majority of bacterial colonists on any given plant have no noticeable effect on plant growth or function.
Estimates suggest that the roughly 1 billion square kilometers of worldwide leaf surfaces host more than 10^26 bacteria, which are the most abundant colonizers of this habitat (2). Overall, microbiota in this ecosystem are large enough to have an impact on both global carbon and nitrogen cycles. Further more the phyllosphere mircroorganisms influence their hosts at the level of the individual plants.
With repeated and rapid constant change of environmental conditions occurring on leaf surfaces, the phyllosphere is recognized as a hostile environment to bacteria. Leaf surfaces are often dry and temperatures can reach 40–55°C under intense sunlight. During the night, however, leaves are frequently wet with dew and at cool temperatures (5–10°C) (2).
Microbes that live in the Phyllosphere are called [Epiphytes]
Physical environment
The leaf surface is exposed to rapidly changing temperature and relative humidity. Also the repeated alternation between presence and absence of moisture due to rain and dew (3,6). The leaf also provides limited nutrient resources to bacterial colonists. The rapid and severe changes in the physical conditions of the phyllosphere creates a hostile microbe environment.
Several factors may influence the microhabitat experienced by bacteria on leaves. First, the leaf itself is surrounded by a very thin laminar layer in which moisture emitted through stomata may be sequestered, alleviating the water that stress epiphytes (4).
Second, some cells in a leaf bacterial population, particularly in [plant-pathogenic] populations locally invade the interior of the leaf, avoiding the stresses on the exterior of the leaf by residing in substomatal chambers or other interior locations. While some phytopathogens have the option of avoiding stresses, most epiphytes must tolerate them in some way (Lindow, S).
Epidermal cells produce hills and valleys that will determine the shape and size of low areas on the surface, which will influence the shape and spread of water droplets on the plant. The first contact between immigrating bacteria and a leaf normally occurs at the plant cuticle. The waxy layer, which has different three-dimensional crystalline structures on different plant species and can change as leaves age,is partially due to microbial modifications. These modifications limit passive diffusion of nutrients and water vapor from the plant's interior onto the surface and defines the hydrophobicity of the leaf. Thick waxy cuticles have thus been thought to interfere with bacterial colonization of plants by limiting diffusion of nutrients and inhibiting the wetting of the leaf surface (Lindow, S).
Because the Phyllosphere is a hostile environment for the residing microorganisms physical parameters contribute to stressful conditions, such as UV radiation, temperature shifts, and the presence of reactive oxygen species. Adaptation to stressful conditions was reflected by the detection of various proteins, assigned to diverse bacterial genera and detected in all analyzed samples. Among these proteins were superoxide dismutase, catalase, DNA protection proteins, chaperones, and proteins involved in the formation of the osmoprotectant trehalose (1).
Biological interactions
There is evidence that bacteria form large and heterogeneous aggregates on plant surfaces. Microscopic examinations of colonized leaves revealed that on plant surfaces, many epiphytes occur in large mixed-bacterial-species aggregates that also harbor fungi. While large numbers of solitary bacterial cells occur on plants, a few large masses of apparently mixed bacterial species can be found.
Such aggregates can constitute between 30 and 80% of the total bacterial population on certain plant species. These assemblages, with an extent and structure similar to those of biofilms that develop in aquatic habitats, are probably found only on long-lived leaves in moist climates such as the tropics or wet temperate regions such as the Pacific Northwest.
The conglomerates on most other plants, while still sizable, are best known as aggregates. The formation of aggregates by bacteria on plants has major implications for the ability of these microbes to colonize and survive the harsh environment of the phyllosphere (8,9); it may provide them with a means to modify their immediate environment in the habitat . The production of extracellular polysaccharide (EPS), which is considered to form a major part of the bacterial aggregate matrix, may benefit epiphytes in the phyllosphere
to see a photo of phyllospheric bacteria click the link [microbial interactions]
Given that water availability is likely one of the most highly fluctuating factors on leaf surfaces, the heavy EPS slime within aggregates may shield the bacteria from desiccation stress by buffering the matric and osmotic potentials of their surroundings. Additionally, EPS has a role in protecting plant-associated bacteria from reactive oxygen species, which are often encountered on plants. It has been demonstrated that aggregated bacteria resist oxidative stress better than planktonic bacteria (1,7).
Microbial processes
There is viable evidence that bacteria form large and heterogeneous aggregates on plant surfaces. Microscopic examinations of colonized leaves revealed that on plant surfaces, many epiphytes occur in large mixed-bacterial-species aggregates that also harbor fungi . While large numbers of solitary bacterial cells occur on plants, a few large masses of apparently mixed bacterial species can be found.
Subsection 1
Such aggregates can constitute between 30 and 80% of the total bacterial population on certain plant species. These assemblages, with an extent and structure similar to those of biofilms that develop in aquatic habitats, are probably found only on long-lived leaves in moist climates such as the tropics or wet temperate regions such as the Pacific Northwest.
Subsection 2
The conglomerates on most other plants, while still sizable, are best known as aggregates. The formation of aggregates by bacteria on plants has major implications for the ability of these microbes to colonize and survive the harsh environment of the phyllosphere (8,9); it may provide them with a means to modify their immediate environment in the habitat . The production of extracellular polysaccharide (EPS), which is considered to form a major part of the bacterial aggregate matrix, may benefit epiphytes in the phyllosphere
to see a photo of phyllospheric bacteria click the link [microbial interactions]
Subsection 2a
Given that water availability is likely one of the most highly fluctuating factors on leaf surfaces, the heavy EPS slime within aggregates may shield the bacteria from desiccation stress by buffering the matric and osmotic potentials of their surroundings. Additionally, EPS has a role in protecting plant-associated bacteria from reactive oxygen species, which are often encountered on plants. It has been demonstrated that aggregated bacteria resist oxidative stress better than planktonic bacteria (1,7).
Key Microorganisms
The study of bacterial colonizers of leaves has been restricted mostly to aerobic culturable bacteria and also driven by the importance of investigating the ecology of plant-pathogenic bacteria because of their deleterious effect on plant productivity. Thus, the microbial ecology of the phyllosphere has been viewed mainly through the biology of gram-negative bacteria such as Pseudomonas syringae and Erwinia (Pantoea) spp., two of the most ubiquitous bacterial participants of phyllosphere communities.
In recent years, the association of multiple outbreaks of food-borne illness with fresh fruits and vegetables has raised concern about the possible preharvest contamination of plants with human pathogens. Surveys of the occurrence of enteric pathogens on produce showed that Salmonella spp. and Shigella spp. were detected in up to 4% of the samples (2).
Two independent studies have shown that [Salmonella enterica] and [Escherichia coli] have the ability to colonize corn, bean, and cilantro plants under humid conditions, albeit to lower population levels than those of common bacterial epiphytes. Unlike P. syringae, they failed to grow on leaves under dry conditions. However, S. enterica survived dry conditions on cilantro leaves and recovered to achieve significant population sizes under subsequent wet conditions (4,6). The relative fitness of some human enteric pathogens in the phyllosphere, as well as the wide distribution on plants of Enterococcus spp. and of common opportunistic pathogens such as Pseudomonas aeruginosa and Burkholderia cepacia, encourage technological innovation in the field of crop harvesting and food safety with regards to virus's.
There are several microbes that live on the Phyllosphere which are of great importance in regards to humans. These microbes account for a large amount of the food borne illnesses in the United States: Campylobacter,Escherichia coli O157:H7, Salmonella, [Cryptosporidium], [Toxoplasma] and [Cyclospora]. A large amount of the food crop regulatory commissioning focus's on eliminating these bacteria and protozoa.
Phyllosphere bacteria can promote plant growth and both suppress and stimulate the colonisation and infection of tissues by plant pathogens. Fungal endophytes of leaves may deter herbivores, protect against pathogens and increase drought tolerance. Interactions in the phyllosphere zone determine the extent to which human pathogens are able to colonise and survive on plant tissues, an area of greatimportance with the rise in cases of human disease associated with consumption of fresh salad, fruit and vegetable produce.
Examples of organisms within the group
There are many organisms which live in the Phyllosphere, they vary from microscopic to several inches in length. The most Common organism in the Phyllosphere is bacteria, mostly bacilli and are not detrimental to the plant, although there are around 100 species that are classified as pathogens and are harmful to the plant or primary consumers.
Fungi, Oomycetes, phytoplasma, nematodes, protozoa and even small parasitic plants have been found in the Phyllosphere.
Current Research
Research into the characteristics of microbial life in the phyllosphere is of great commercial importance to the agricultural industry for two reasons. First, understanding the survival of plant disease-causing bacteria and fungi is vital for developing new ways to control their spread. Second, there has been a recent rise in the number of food poisoning cases associated with fruit and vegetables contaminated with bacteria such as Salmonella and E. coli O157:H7. This is particularly true of fresh fruits and salads which are not cooked prior to consumption. Preventing these outbreaks by developing better decontamination strategies is important to protect public health.
Immediate-term information is needed to guide growers, Cooperative Extension, the diagnostic service industry, shippers, and processors in the development of on-the-farm management practices to prevent these microbial pathogens from being introduced during productionand at harvest. Areas researchers are beginning to address include (4);
· Information on sources and persistence
· Manure management and compost process control
· Timing of incorporation of animal manures relative to crop seeding and harvest
· Depth of incorporation into soil to minimize persistence or transfer
· Potential for establishment of key pathogens on plant parts during production
· Postharvest prevention programs
References
(1) http://aem.asm.org/cgi/content/full/69/4/1875#F1
(2) http://www.pnas.org/content/106/38/16428.full
(3) http://www.iripz.pl/ftp/09_biotic.pdf
(4) http://ucce.ucdavis.edu/files/filelibrary/5453/6558.PDF
(5) http://en.wikipedia.org/wiki/Plant_pathogen#Oomycetes
(6) Sylvia, D. 2007. Principles and applications of soil microbiology
(7) hhttp://www.microbiologyprocedure.com/growth-of-microorganisms/rhizosphere-and-phyllosphere.html
(9) http://wrap.warwick.ac.uk/449/1/WRAP_Bending_JAM_review_revised_4_April_2008.pdf
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.