Prevotella nigrescens
The mouth is the home of a diverse, abundant and complex microbial community. This highly diverse microflora community resides on the various surfaces of the normal mouth. Rather than a passive relationship with the host, these resident microflora play a positive role in normal development of the host. A dynamic equilibrium exists between the normal microflora and the innate host immune system. The host immune system is highly efficient and constantly monitors microflora levels in order to prevent these normally benign resident microflora from invading oral tissues. When certain microflora colonize and increase, they increase the incidence of many diseases including but not limited to oral infections. One specific type of bacteria that is part of the normal oral flora but leads to disease when it infects the local tissue is Prevotella nigrescens.
Prevotella nigrescens (P. nigrescens), a member of the Prevotella species found in normal oral flora, was first discovered within a group of bacteria termed Bacteroides intermedius.[1] The fact that there was heterogeneity within this species, Bacterioides intermedius, had been recognized for some time. This discrepancy led to further biochemical and chemical studies that revealed two serotypes within Bacterioides intermedius, one of which was Prevotella intermedia. It was upon characterization of the newly discovered Prevotella intermedia that revealed yet another profile within this assumed homogenous species, which is what we now know as Prevotella nigrescens.
Following the discovery of these two distinct strains, studies of P. intermedia and P. nigrescens found them to play a role in the pathogenesis of periodontaland endodontic disease. Periodontal disease is a synonym for periodontitis. Periodontitis is a set of inflammatory diseases affecting the tissue that surround and support teeth. P. nigrescens causes periodontitis by colonizing on the tooth surface and triggering an overly aggressive immune response, which is perceived as inflammation of oral tissue. More recently, P. nigrescens has also been found to be associated with signs of carotid atherosclerosis even in patients without periodontitis and with endodontic infections.[2] [3]
As more information on the pathogenesis properties of P. nigrescens was uncovered, there was a demand to find a treatment. Under this pressure a potential alternative treatment, to antibiotics, for P. nigrescens was found in the use of bee glue.[4]
Previous studies suggest that P. intermedia is more often found in periodontal sites whereas P. nigrescens is more frequently found in healthy gingivae. However, P. nigrescens have been implicated as a major periodontal pathogen as well. It is important to note the site specificity and pathogenicity of P. nigrescens remains unclear.[3] [4] Overall, studies up to date show that there are increased levels of P. nigrescens in oral diseases and therefore implicating P. nigrescens as an oral pathogen but further research remains to be conducted to confirm these findings.
Discovery
Differentiation from P. intermedia
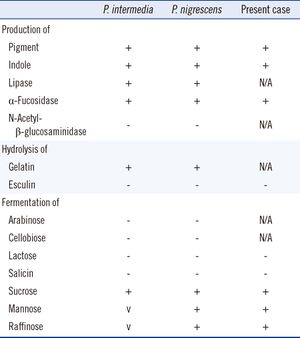
Identification of samples as either P. intermedia or P. nigrescens can be made based on differences in malate and glutamate dehydrogenase electrophoretic mobility. Sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) of whole cell samples show that P. nigrescens have 31 and 38 kDa proteins that are not present in P. intermedia.[5] Surface biotinylation of cells revealed that the 31 kDa protein is on the surface of the protein. Fimbria-like projections were also observed in negatively stained cells of P. nigrescens but not in P. intermedia.[5]
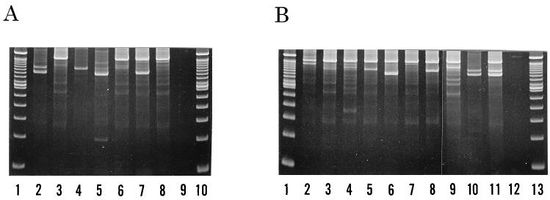
Although no further research has been done on the 38 kDa protein, this protein may be of further help to distinguish both species as it stained strongly only in P. nigrescens. In terms of enzymatic differences, P. intermedia contain fast-moving enzymes whereas P. nigrescens have slower moving enzymes.[3] P. intermedia and P. nigrescens also differ in localization. P. nigrescens is almost three times more frequently isolated from infected root canals than P. intermedia.[3]
However, identifying the two strains using methods such as SDS-PAGE, restriction endonuclease analysis, ribotyping, and arbitrarily primer PCR (AP-PCR) are either time-consuming or have low levels of reproducibility. A newer method, PCR ribotyping, is expected to amplify the 16S and 23S spacer regions of bacterial rRNA genes by PCR. [6] It generates simple banding patterns that allows comparison between separate PCR amplifications. Since PCR ribotyping amplicons smaller than 600bp were poorly reproduced, higher discriminatory assays were run by using a combination of PCR ribotyping and AP-PCR fingerprinting. The combination assay identified seven types within the P. intermedia strain and ten types within the P. nigrescens strain, all of which were differentiable by a combination of their band patterns and DNA fingerprints.
Cellular morphology and biochemistry
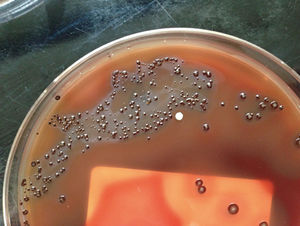
P. nigrescens are black-pigmented Bacteroides that ferment carbohydrates. They are gram-negative, non-sporing, obligatory anaerobic, rod-shaped bacterium of the genus Prevotella that is commonly found in oral flora.
Cells grow in broth cultures are 0.3 to 0.7 µm wide by 1 to 2 µm. In agar, colonies are 0.5 to 2 mm in diameter, circular, low convex and smooth. In blood agar, the colonies are brown to black suggesting weakly hemolytic, and sometimes alpha-hemolytic, properties. Pigmentation is mainly towards the outer edges whereas the center remains creamy to dark brown. Strains grown on blood agar also exhibit red fluorescence under long-wave UV radiation (365nm).[1]
P. nigrescens uses mixed fermentation in anaerobic living conditions, breaking down dextrin, glucose, maltose, and sucrose. In the process of fermentation, they produce acetic, isobutryic, isovaleric, and succinic acids. Characterization using the API ZYM system revealed that P. nigrescens reacted with acid and alkaline phosphatases, phosphoamides, and β-glucosidases. The system showed weak reactions with butyrate esterase and caprylate esterase lipase. The principal respiratory quinones are unsaturated menaquinones. The G+C content of the DNA is 40 to 44 mol%.[1]
Role in Disease
Periodontitis
P. nigrescens is part of the orange complex of subgingival and supragingival plaque. It is also strongly related to bacteria of the red complex, which is the most commonly used periodontal diagnosis and therefore is considered periodontal pathogens. [8] Much evidence supporting its pathogenic role comes from quantitative cultural studies, which is the comparison of certain pathogen levels in diseased sites to healthy sites.[9]
Recently, it was discovered that P. nigrescens is found more frequently in subgingival plaque of patients with periodontitis.[1] Periodontal sites are classified as either active, inactive or healthy using a number of widely accepted clinical criteria.[9] Researchers found a correlation between increased colonization of P. nigrescens and periodontitis.[1] Another group found that up to 70% of patients with localized and generalized forms of onset periodontitis had detectable levels of P. nigrescens in their subgingival plaque.[10] More recently, P. nigrescens was accepted as a putative periodontal pathogen for periodontal disease. Studies suggest that the root of its pathogenicity lies in that it seems to promote the release of inflammatory mediators.[11] [12]
Contradictory data showed that 9 of 21 subjects (15 periodontitis patients and 6 healthy volunteers) harbored more than one genotype of P. nigrescens. Several different types were found occupying different sites around the same tooth.[9] This data suggested that no specific relationship existed between disease status and colonization of same or different types of P. nigrescens.
Endodontic Infections
Carotid atherosclerosis
Carotid atherosclerosis is also known as arteriosclerotic vascular disease. It is marked by the thick of artery walls in the heart, which is a result of invasion and accumulation of white blood cells. However, the developmental process is not well studied. It has been suggested inflammatory processes in the endothelial cells of the vessel walls initiate the onset of disease.[13] Diagnosis involved ultrasound scanning and calculated intimamedia area (cIMA) measurements.
Several studies published in the past two decades have implicated oral diseases, periodontitis in particular, as risk factors for the development of cardiovascular diseases.[2] Cardiovascular disease (CVD) is a class of disease that involves the heart or blood vessels. A few examples of CVD include coronary thrombosis, stroke and atherosclerosis. The way through which periodontitis contributes is by increasing the systemic inflammatory burden and pathogenic processes that are known to lead to atherosclerosis.[14] More recently, a direct association was established between the localization of P. nigrescens in healthy oral cavity and atherosclerosis.[2]
P. nigrescens is characterized as a periodontal pathogen. It contributes to the infection and inflammation of periodontal tissue, which are characteristics commonly associated with periodontitis. However, since periodontal pathogens are not sufficient in the pathogenesis of periodontitis P. nigrescens has been observed in healthy oral flora as well. Recent study showed an association of increased cIMA with the presence of P. nigrescens.[2] It has been hypothesized that P. nigrescens is released from periodontal lesions into circulation. There, it affects the arterial wall by virulence compounds (such as lipopolysaccharides), invades epithelial cells, inducing mechanisms that are detrimental to the host cells and thus causing an accumulation of white blood cells leading ultimately to carotid atherosclerosis.[2]
Treatment
Bee Glue
The development of new therapies for treatment of oral cavity diseases is of great importance since the development of antibiotic resistance of certain microbes. One of these new treatments that have emerged is the use of propolis, more commonly known as bee glue.[4]
The biological activities of bee glue include antibacterial, antifungal, antiprotozoan, antiviral, antitumoral, immunomodulation, anti-inflammatory and other therapeutic properties.[4] It has been shown to be effective in treating gum infections due to P. nigrescens. Penicillin G, erythromycin, meropenem, metronidazole and tetracycline were found to inhibit 90% of the growth (MIC90) of P. intermedia and P. nigrescens strains at of 0.06, 0.13, 2.0, 4.0 and 4.0ug/mL respectively. iP. intermedia and P. nigrescens were found to be susceptible to three ethanolic extract of propolis at 128ug/mL.[4]
Future directions
References
1. Shah HN, Gharbia SE (1992). Biochemical and chemical studies on strains designated Prevotella intermedia and proposal of a new pigmented species, Prevotella nigrescens sp. nov. Int J Syst Bacteriol 42: 542–6.
2. Yakob M., Söder B., Meurman J. H., Jogestrand T., Nowak J., Söder P.-Ö. (2011). Prevotella nigrescens and Porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontitis. Journal of Periodontal Research 46 (6): 749–755.
3. Saheer E.G, Markus H, Haroun N.S, Anja K, Kari L, Michelle A. P., Deirdre A. D. (1994) Characterization of Prevotella intermedia and Prevotella nigrescens Isolates From Periodontic and Endodontic Infections. Journal of Periodontology 65(1): 56-61
4. Santos, F. A., Bastos, E. M. A., Rodrigues, P. H., de Uzeda, M., de Carvalho, M. A. R., de Macedo Farias, L., Moreira, E. S. A. (2002). Susceptibility of Prevotella intermedia/Prevotella nigrescens (and Porphyromonas gingivalis) to propolis (bee glue) and other antimicrobial agents. Anaerobe, 8(1), 9-15.
5. Fernández-Canigia L, Cejas D, Gutkind G, Radice M. (2015) Detection and genetic characterization of β-lactamases in Prevotella intermedia and Prevotella nigrescens isolated from oral cavity infections and peritonsillar abscesses. Anaerobe. 2015 Jan 23;33C:8-13.
6. Fukui K, Kato N, Kato H, Watanabe K, Tatematsu N (1999). Incidence of Prevotella intermedia and Prevotella nigrescens carriage among family members with subclinical periodontal disease. Journal of Clinical Microbiology 37 (10): 3141–5.
7. Newman , M.G (2011). Carranza's Clinical Periodontology, 11th Edition. Saunders Book Company: p.245
8. Stingu C.S., Schaumann R., Jentsch H., Eschrich K., Brosteanu O., Rodloff A.C (2013). Association of periodontitis with increased colonization by Prevotella nigrescens. Journal of Investigative and Clinical Dentistry 4 (1): 20–25.
9. Teanpaisan R, Douglas CW, Eley AR, Walsh TF (1996) Clonality of Porphyromonas gingivalis, Prevotella intermedia and Prevotella nigrescens isolated from periodontally diseased and healthy sites. Journal of Periodontal Research 31(6): 423-432
10. Mullally BH, Dace B, Shelburne CE, Wolff LF, Coulter WA. (2000) Prevalence of periodontal pathogens in localized and generalized forms of early-onset periodontitis. J Periodontal Res 35: 232–41.
11. Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ (2007). Activation of toll-like receptors 2 and 4 by Gram negative periodontal bacteria. Oral Microbiol Immunol 22: 145–51.
12. Airila-Mansson S, Soder B, Kari K, Meurman JH (2006). Influence of combinations of bacteria on the levels of prostaglandin E2, interleukin-1ß, and granulocyte elastase in gingival crevicular fluid and on the severity of periodontal disease. J Periodontol 77: 1025–31.
13. Williams KJ, Tabas, I (1995) The Response-to-Retention Hypothesis of Early Atherogenesis Arteriosclerosis, thrombosis, and vascular biology 15 (5): 551–61.
14. Tonetti Ms (2009) Periodontitis and risk for atherosclerosis: an update on intervention trials J Clin Periodontol 36: 15-19.
15. Yang JJ, Kwon TY, Seo MJ, Nam YS, Han CS, Lee HJ. (2013) 16S Ribosomal RNA Identification of Prevotella nigrescens from a Case of Cellulitis. Ann Lab Med 3(5):379-382.
Edited by Nancy Zhu, a student of Suzanne Kern in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.
