Saccharopolyspora spinosa: Difference between revisions
| Line 36: | Line 36: | ||
<b>Metabolism</b><br> | <b>Metabolism</b><br> | ||
''Saccharopolyspora spinosa'' is a chemoorganoheterotroph that decomposes organic matter to produce energy. It may utilize a list of organic acids for carbon and energy. Some of these compounds include: acetate, butyrate, citrate, formate, lactate, malate, proprionate, pyruvate, and succinate (Mertz and Yao 1990). This species | ''Saccharopolyspora spinosa'' is a chemoorganoheterotroph that decomposes organic matter to produce energy. It may utilize a list of organic acids for carbon and energy. Some of these compounds include: acetate, butyrate, citrate, formate, lactate, malate, proprionate, pyruvate, and succinate (Mertz and Yao 1990). This species also reduces nitrogen. During metabolism ''S. spinosa'' generates compounds such as: _________ . These compounds are mainly generated in a production setting for the purpose of extracting desired compounds for the production of active ingredients in pesticides.It is important to note that the metabolism of ''S. spinosa'' has only been observed in laboratory and production settings.<br> | ||
<b>Life Cycle</b><br> | <b>Life Cycle</b><br> | ||
Revision as of 22:12, 15 April 2012
UNDER CONSTRUCTION
Classification
Domain: Bacteria
Phylum: Actinobacteria
Class: Actinobacteria
Order: Actinomycetales
Family: Pseudonocardiaceae
Genus: Saccharopolyspora
Species
Saccharopolyspora spinosa
|
NCBI: [1] |
Related species:
Saccharopolyspora hirsuta
Saccharopolyspora erythraea
Description and Significance
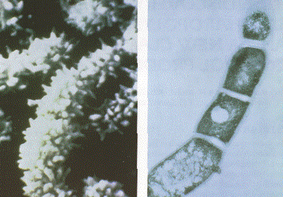
In 1982, researchers vacationing in the Virgin Islands discovered the actinomycete bacteria, Saccharopolyspora spinosa, in soil collected at an inoperative sugar mill rum still (Mertz and Yao 1990, Thompson et al. 2000). Similar to other species in the Saccharopolyspora genus, S. spinosa are aerobic, gram-positive, and have mycelium (Mertz and Yao 1990). The mycelium are made up of pale yellowish-pink aerial hyphae, and yellow to yellow-brown substrate hyphae (Mertz and Yao 1990). These hyphae are long bead-like spore chains (with 50+ spores) that are surrounded by spiny (='spinosa') spore sheaths (Mertz and Yao 1990). Spores are oblong and approximately 1.1μm by 1.5μm (Mertz and Yao 1990). Aerial hyphae have a hook, loop and incomplete spiral arrangement that are described as 'rectus-flexibilius' (Mertz and Yao 1990). These bacteria are most similar to S. hirsuta and S. erythraea in morphology, but have different physiological characteristics compared to other species in this genus (Mertz and Yao 1990). Furthermore, since the discovery of S. spinosa, many research cultures have developed different strains of S. spinosa that have distinct characteristics. Strains of S. spinosa are convoluted in scientific literature, but a few including the type strain: A83543.1 are listed on StrainInfo.net.
Saccharopolyspora spinosa produce metabolites named 'spinosyns' that are economically, ecologically, and agriculturally important. It has been found that spinosyns have pesticidal properties that are effective for many mites and insects (Salgado 1998, Bond et al. 2004, Cloyd 2009, Thompson et al. 2000, Watson 2001, Hertlein et al. 2011). Interestingly, the discovery of these properties led to a patent on S. spinosa and the byproducts produced by the organisms (Turner et al. 1995). Spinosyns are generated by S. spinosa in an aerobic fermentation broth consisting of necessary metabolic nutrients and the S. spinosa organisms. The spinosyns extracted from the broth make up a class of insecticides that are sold under trade names such as Conserve® and Entrust®. Because the active ingredients in these pesticides are biological byproducts, the pesticides are marketed as "natural," and less toxic compared to other available chemicals. Furthermore, the spinosyns have two modes of action that help prevent development of resistances. Therefore, pesticides containing spinosyns are attractive options for pest management in agriculture.
Genome Structure
The genome of Saccharopolyspora spinosa has been drafted by the Beijing Genomics Institute. It was produced using a genome shotgun strategy as well as pyrosequencing. The entire genome was found to be at 8,581,920 base pairs with 8,302 predicted coding sequences. G+C content was found to be 67.94% (Pan et al. 2011). This organism is still under study and its genome is in the process of being fully mapped.
Cell Structure, Metabolism and Life Cycle
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Cell Structure
The cell wall of Saccharopolyspora spinosa is mainly composed of a thick layer of peptidoglycan, and lipoteichoic acids, that serve as chelating agents and also for adherence. The individual peptidoglycan molecules are cross-linked by pentaglycine chains using a DD-transpeptidase enzyme. Spores of S. spinosa are round to oval or oblong and covered by a sheath. The cell wall of this organism, just as with other members in its genus is characterized by the absence of mycolic acids (long-chain α-substituted Β-hydroxylated fatty acids) (Alexander et al. 2000).
Metabolism
Saccharopolyspora spinosa is a chemoorganoheterotroph that decomposes organic matter to produce energy. It may utilize a list of organic acids for carbon and energy. Some of these compounds include: acetate, butyrate, citrate, formate, lactate, malate, proprionate, pyruvate, and succinate (Mertz and Yao 1990). This species also reduces nitrogen. During metabolism S. spinosa generates compounds such as: _________ . These compounds are mainly generated in a production setting for the purpose of extracting desired compounds for the production of active ingredients in pesticides.It is important to note that the metabolism of S. spinosa has only been observed in laboratory and production settings.
Life Cycle
Many actinomycetes are soil dwelling decomposers (##ref). S. spinosa was discovered in soil and has been kept in culture... It has not been found in nature since its discovery (ref).
Ecology and Pathogenesis
Habitat
Saccharopolyspora spinosa is kept in culture. Culture medias that have demonstrated good growth conditions for S. spinosa include actinomycete isolation agar, Bennett agar, tomato paste-oatmeal agar (Waksman 1961 via Mertz and Yao 1990, Mertz and Yao 1990), and BHI (Brain Heart Infusion) agar (Waldon et al. 2001). The optimal temperature for growth in media are 15-37°C (Mertz and Yao 1990). These organisms are also grown in fermentation broths (Waldon et al. 2001).
Ecological, Environmental, and Applied Significance
The metabolites produced by S. spinosa make up the low-risk insecticidal chemical called Spinosad. This natural pesticide consists of a mixture of tetracyclic macrolide neurotoxins, spinosyn A and D that kills many pests and has no known toxic effect on humans (Pan et al. 2011, Bond et al. 2004). This chemical is used in several countries to protect stored grain from pests. Spinosad is effective for large range of insects, predominantly moths and beetles. It is also being used as a larvicide for in the control of container breeding mosquitoes, an attractive option due to its highest ratings for safety, environmentally favourable toxicity profile and low persistence (Bond et al. 2004). This chemical compound is able to remain viable in dry grain storage bins and its length of virility has been measured to last from six months up to two years. The effectiveness of this compound is still being explored and it is reported that its strength is determinant upon several factors such as: insect species, pest life stage, grain type, and grain variety. Spinosad has also been marketed as a treatment for headlice under the pharmaceutical name Natroba.
Mode of Action of Spinosyns
Spinosyns have a unique mode of action involving the postsynaptic nicotinic acetylcholine and GABA receptors (Bond et al. 2004, Watson 2001). Spinosyn A, the major active component of spinosad, causes involuntary muscle contractions and tremors by widespread excitation of neurons in the central nervous system of insects at low concentrations. Prolonged spinosyn-induced hyperexcitation results in paralysis associated with neuromuscular fatigue and death (Salgado, 1998).
Symbiosis
The interrelationships between S. spinosa and other organisms has not been thoroughly investigated. However in a study using potato cyst nematode, Globodera rostochiensis, bioagents obtained from S. spinosa caused significant inhibitory effect on multiplication of the nematode, suggesting a possible antagonistic relationship with this organism in nature (Trifona 2010).
Environmental significance
The discovery of S. spinosa has led to the realization of a novel family of unprecedented environmentally friendly compounds with many applications in modern integrated pest management programs. Owing to their relatively selective activity against target insects in comparison to many other insecticides, coupled with generally lesser activity against many beneficial insect predators and other non-target species, spinosyns are proving to be choice compounds in pest control. Furthermore, spinosyns posses a desirable attribute of low toxicity and reduced risk toward mammals and many other aquatic and avian fauna (Kirst 2010). Chronic studies have demonstrated that spinosad was not carcinogenic, mutagenic or terotogenic to mammmals (Kirst 2010, Thompson et al. 2000). These compounds do not have a relative long period of persistence in the environment and are easily degraded by sunlight- based photolytic processes, and partitioned into organic and sediments with subsequent microbial degradation in the absence of light (West 1997, Thompson et al. 2002, Kirst 2010).
References
Alexander, M., Bloom R.B., Hopwood, D.A., Hull, R., and B.H., Iglewski (Ed). Encyclopedia of Microbiology. Second Edition. 2000. Elsevier Science.
Bond J.G., Marina, C.F., and Williams, T. "The naturally Derived Insecticide Spinosad is highly Toxic to Aedes and Anopheles Mosquito Larvae. Medical and Veterinary Entomology. 2004. Volume 18. p 50-56
Cloyd, R. "Western Flower Thrips Managment: Have We Reached An Impasse?". OFA Bulletin. 2009. Volume 918. p. 24-29.
Hertlein Mark B. et al. "Spinosad: A New Natural Product for Stored Grain Protection". Journal of Stored Products Research. 2011. Volume 47. p. 131-146.
Kirst, H.A. "The spinosyn family of insecticides: realizing the potential of natural products research". The Journal of Antibiotics. 2010. Volume 63. p 101–111.
Mertz, F.P., and Yao, R.C. "Saccharopolyspora spinosa sp. nov. Isolated from Soil Collected in a Sugar Mill Rum Still". International Journal of Systematic Bacteriology. 1990. Volume 40(1). p. 34-39.
Pan, Y., Yang, X., Li, J., Zhang, R., Hu, Y., Zhou., Y., Wang, and B Zhu . "Genome Sequencing of the Spinosyns-Producing Bacterium Saccharopolyspora Spinosa NRRL 18935". Journal of Bacteriology. 2011. Volume 193(12). p. 3150-3151.
Salgado, V.L. "Studies on the Mode of Action of Spinosad: Insect Symptoms and Physiological Correlates". Pesticide Biochemistry and Physiology. 1998. Volume 60. p 91–102.
Thompson, G.D., Dutton, R., and Sparks, T.C. "Spinosad - a case study: an example froma natural products discovery programme". Pest Management Science. 2000. Volume 56. p. 696-702.
Thompson, D. G., Harris, B. J., Lanteigne, L. J., Buscarini, T. M. & Chartrand, D. T. "Fate of spinosad in litter and soils of a mixed conifer stand in the Acadian forest region of New Brunswick". J. Agric. Food Chem. 2002. Volume 50 790–795.
Trifonova, Z.T. "Studies on the Efficacy of some Bacteria and Fungi for Control of Globodera restochiensis". Journal of Agricultural Sciences. 2010. Volume 55 (1). p. 37-44.
Turner, J.R., Huber, M.L.B., Broughton, M.C., Mynderse, J.S., Martin, J.W.
Waldon, C., Matsushima, P.,Rosteck, P.R.J., Broughtonb, M.C., Turnerb, J., Madduria, K., Crawforda, K.P., Merloa, D.J., and Baltz, R.H. "Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa". Chemistry & Biology. 2001. Volume 8(5). p. 487–499.
Watson, G.B. "Actions of Insecticidal Spinosyns on γ-aminobutyric Acid Receptors from Small Diameter Cockroach Neurones". Pesticide Biochemistry and Physiology. 2001. Volume 71. p. 20–28.
West, S. D. "Determination of the naturally derived insect control agent spinosad and its metabolites in soil, sediment, and water by HPLC with UV detection". J. Agric. Food Chem. 1997. Volume 49. p. 3107–3113.
Author
Page authored by Emily Pochubay, Matt O'Grady, Placid Mpeketula, and Demetrious Parker students of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->