Salmonella enterica serovar Typhi

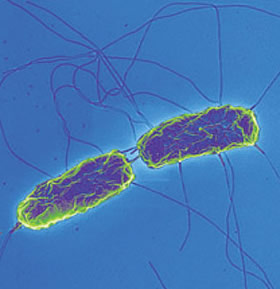
Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gammaproteobacteria
| Order = Enterobacteriales
| Family = Enterobacteriaceae
| Genus = Salmonella
| species = S. enterica
| serotype = Typhi
Description
Salmonella enterica serovar Typhi is a gram-negative facultative anaerobe. While this bacterium strictly infects humans, scientists remain uncertain regarding the reason behind this pathogen’s selective host behavior. This rod shaped invasive pathogen initially propagates inside the intestinal tract and spreads throughout the peripheral lymphatic system, such as the bone marrow and Peyer’s patch, to cause typhoid fever [3]. Typhoid fever generally occurs in four stages throughout a four-week period with an incubation period of 1 to 2 weeks before the onset of initial symptoms. The incubation period may vary depending on the host immune system and severity of infection. Beginning with a fever between 103-104°F, the patient's condition will continue to deteriorate due to symptoms including diarrhea, rashes, and delirium. Patient death is largely the result of complications from the illness including myocarditis, intestinal bleeding or perforations [5].
This human pathogen has existed for thousands of years by thriving in poor sanitary conditions, especially in crowded areas. Some scientists and historians have suggested that this bacterium may have been responsible for the Plague of Athens in the final stages of the Pelopennesian War. In conjunction, Salmonella Typhi is derived from the ancient Greek word typhos, defining an ethereal smoke or cloud believed to cause madness and disease. Antibiotics have reduced the frequency of typhoid fever, yet it still remains endemic in developing countries. The relatively new syndrome, S. paratyphi causes similar symptoms. Some populations have labeled it as the same strain because of ignorance in populations and incomplete coverage from vaccines targeting typhi. Both S. Typhi and S. Paratyphi serovars are referred to collectively as typhoidal salmonella.
Typhoid fever is usually treated with antibiotics such as Ciprofloxacin. Due to the use of fluoroquinolones, over 10 years the clonal expansion of the halotype H58 has increased in Asia and Africa where the invasive Salmonella Typhi continues to constitute a severe risk. However, other strains and halotypes of typhoid fever remain sensitive to antibiotics despite the growing selection for antibiotic resistant strains. Additional experimentation and research regarding Salmonella Typhi is difficult due to its preference for human hosts. Mouse models infected with S. enterica serovar Typhimurium have been the main source to observe pathogenic effects due to its similar genome, which only differs by 11%.
Pathogenesis
Transmission
The transmission of Salmonella enterica serovar Typhi, like most Samonella serovars, occurs through the fecal-oral method. The pathogen is human host-adapted, and generally spreads through contaminated food and water sources. Some hosts of Salmonella Typhi become asymptomatic carriers and can unknowingly transmit the bacteria to others. Because they are unaware of their condition, these carriers often lack proper preventative measures for slowing the spread of bacteria. Without these preventions, the bacteria are able to attain a high transmission rate. While lacking symptoms themselves, these carriers excrete large amounts of the bacteria in their feces and pass on the pathogen by contaminating food and water sources. [4] A primary example of asymtomatic carriers causing high transmission rate is that of "Typhoid Mary." Mary Mallon, an Irish cook in the New York City area, was the first known typhoid carrier in the United States. Despite her complete lack of symptoms, it is believed that Mallon caused a minimum of seven outbreaks of typhoid fever, resulting in 57 cases of the disease and 3 deaths. [4]
Infectious dose, incubation, and colonization
The infectious dose is 10^3-10^6 bacilli, and the incubation period is 7-14 days.
A high infectious dose of Salmonella Typhi is needed for infection because it lacks acid tolerance. Because of this, much of the bacterium is destroyed as it passes through the highly acidic environment of the stomach. The bacterium must be abundant enough to overcome conditions of the stomach to successfully infiltrate the host.
To colonize, S. typhi adheres to the mucosal lining of the small intestine and penetrates the epithelial cells. The bacterium spreads to the peripheral lymphoid organs during secondary infection. The gallbladder serves as the primary reservoir of chronic infection. The formation of biofilms in the gut and on gallstones is a critical factor in the carriage and shedding of S. typhi. [6]
Epidemiology
This disease is a major factor in countries that lack access to purified water, such as India, South America, Southeast Asia, and Africa. Children that play in sewage water on the streets in these regions are at an especially high risk. 80% of cases come from Bangladesh, India, China, Indonesia, Nepal, Laos, Vietnam, or Pakistan. [5] There are more than 27 million cases annually, and 217 thousand deaths world-wide each year. [6]
Virulence Factors
Most of the bacterium's virulence arises from Salmonella pathogenicity islands, also known as SPI. These islands encode for the majority of effector molecules associated with pathogen virulence. For example after entering a host cell, Salmonella. typhi will secrete effector proteins including SIPA and SptP. These proteins will alter the actin cytoskeleton of the host cell, which is responsible for cell migration.
SPI7 is considered the most important pathogenicity island because it codes for the Vi antigen, which is expressed on the surface. This antigen resides within a polysaccharide capsule which is essential for increased virulence and severity of symptoms. It is believed that this capsule also prevents lipopolysaccharide recognition by pattern recognition receptors in order to prevent immune response, however further research is required. Secretion of the protein invasin will allow non-phagocytic cells to ingest the bacterium in order to allow intracellular access leading to the inhibition of oxidative leukocytes, and rendering the innate immune response ineffective. Other possible factors include ion transporters, fimbrae, and flagella required for attachment and colonization. [7]
Recently, the typhoid toxin has been discovered. This toxin is known as chimaeric A2B5 typhoid toxin and is made up of two subunits including the PltA and CdtB. These two subunits are comparable to pertussis and cytolethal and pertussis toxins. It is believed that this toxin is the cause of the high fevers present during the first and second week of infection. [8]
Clinical Features
Acute gastroenteritis is the most common symptom in infected patients. This causes diarrhea, abdominal cramping, fever, and vomiting. Fever will usually subside in 72 hours, with bloody diarrhea lasting between three and seven days. Symptoms of the infection can be classified according to a time frame after exposure.
The first week is characterized by high fevers, fatigue, dry cough, and diarrhea.
If the patient has not received treatment by the second week, symptoms will increasingly worsen including the fever and continuation of diarrhea leading to extreme weight loss.
By the third week with no medical attention, the patient will become delirious and experience severe exhaustion known as the typhoid state. Approximately 5% of people with typhoid fever develop a perforated intestine causing internal bleeding, which could cause blood to appear in the stool. This results in a hole in the small or large intestine, triggering possible symptoms of abdominal pain, nausea, vomiting, and blood sepsis. Other complications associated with typhoid fever include myocarditis, kidney or bladder infections, and meningitis. Psychiatric problems such as delirium, hallucinations, and paranoid psychosis can also occur as a result of infection.
If the patient is able to survive to the fourth week, they will gradually improve and are able to regain their mental state. [5]
These effects can be more severe or prolonged in children and the elderly. Bacteremia, or the spread of the pathogen into the blood stream, generally occurs in 5-10% of cases and can lead to more severe symptoms such as meningitis and infections of the bones and joints. This can be especially dangerous in immunocompromised patients such as those suffering from HIV or Malaria [7].
Diagnosis
Stool cultures are used to diagnose the disease, but in many cases, blood cultures must also be used to reach a confident diagnosis due to the sensitivity of the stool cultures in the early and late stages of illness. The blood cultures from the infected host can be tested on MacConkey agar and EMB agar. [8] [9]
The Salmonella Typhi bacteria enter the bloodstream upon infection and are carried by white blood cells to the liver, spleen, and bone marrow where they multiply in the cells of these organs and then enter back into the bloodstream. As the bacteria invade the lymphatic tissue of the bowel, they continue to proliferate. The bacteria then enter the intestinal tract and can be used in the diagnosis of stool cultures from the laboratory. In early and late stages of the disease, stool cultures are more sensitive to culturing, but should be tested simultaneously with blood culture to make a definitive diagnosis.
Additional testing may be done to differentiate between the different serovars of S. enterica species. These molecular biological tests are based on different antigens on the bacteria's surfaces, such as O, K, and H. [3]
Treatment
Antibiotic therapy, specifically ciprofloxacin and ampicillin, are the only effective treatment. For pregnant women ceftriaxone is used. Recently, antibiotic resistance by this bacterium is increasing and developing into a more serious issue concerning the effectiveness and use of antibiotics. To stimulate recovery, fluids and a healthy diet can be administered in addition to antibiotics. [9]
Previously, antibiotic treatment for typhoid fever included regimens of ampicillin, trimthoproim-sulfamethoxazole, and chloramphenicol. Because of developing drug resistance over the past twenty years, the uses of these drugs is now limited. Strains specific to South America have shown significant resistant to antibiotic therapy. Currently quinolone, macrolide, and third-generation cephalosporin antibiotics are used to treat resistant strains. Quinolone sensitivity has steadily declined in different parts of the world, but it remains significant in the United States.
To treat the carrier state, prolonged antibiotics are prescribed. Additionally, the direct site of chronic infection can be removed, such as gall bladder removal.
Prevention
To avoid infection, hygiene such as clean hands and treated water is encouraged. Boiling water and correct procedure when handling raw fruits and vegetables decrease the risk of infection. [10] In association, two vaccines are available: inactivated typhoid vaccine administered via injection and live typhoid vaccine administered orally. [4]
Host Immune Response
Salmonella enterica serovar Typhi can cause life-threatening bacterial infections called typhoid fever. The uncontrolled activation of the host innate immune response can potentially lead to systematic inflammation, tissue injury, intravascular coagulation, and even death.
Salmonella enterica serovar Typhi is an invasive pathogen. It is recognized by the host’s immune system using toll-like receptors (TLRs), which initiate the innate immune response. The TLRs recognize pathogen-associated molecular patterns (PAMPS) located on the surface of the pathogen. This recognition allows for the innate immune system to initiate its response, which causes the activation and recruitment of macrophages, neutrophils, and cytokines. For example, IFN-γ is a significant cytokine for the triggering of macrophages and early host resistance of Salmonella enterica serovar Typhi.
Victims of typhoid fever are susceptible to reinfection because of the initial infection’s severe disruption of the gut microbiome. A typical host contains a microbiome of 1x10^14 bacteria with an average of 500 to 1,000 different species. A healthy microbiome can protect the host’s epithelial cells from infection. The gut microbiome produces toxic metabolites that can suppress the virulence of Salmonella enterica serovar Typhi’s gene expression, boost the host’s immune response, and help clear the intestinal lumen after non-typhoidal diarrhea. Additionally, apoptosis can strengthen the host’s defense by allowing the body to prevent further release of pro-inflammatory cell mediators. Salmonella enterica serovar Typhi’s ability to use a microbiome nutrient called ethanolamine allows it to colonize in the intestinal tract. This rich nutrient often allows Salmonella enterica serovar Typhi to outcompete other pathogens. Antimicrobial treatment for Salmonella enterica serovar Typhi may cause depletion of the host’s gut microbiome. This depletion can lead to prolonged effects of intestinal colonization, and an increase in carrier status and fecal shedding.
References
1 University of Oklahoma Faculty and Staff.
2 Kenyan Waterborne Disease Center
3 "Salmonella Typhi" Salmonella Typhi.
4 "Typhoid Fever" Center for Disease Control and Prevention.
5 "Diseases and Conditions: Typhoid Fever" Mayo Clinic.
6 de Jong, H.K., Parry, C.M., van der Pol, T. "Host-Pathogen Interaction in Invasive Salmonellosis" PLOS Pathogens.
7 Brusch, J.L. "Typhoid Fever" Medscape.
8 Song, J., Gao, X., Galan J.E. "Structure and Function of the Salmonella Typhi chimaeric A2B5 typhoid toxin" Nature: International weekly journal of science.
9 Pollack, D.V. "Salmonella enterica Typhi" University of Connecticut.
10 Balentine, J.R. "Typhoid Fever" Medicine Net.
11 "Typhoid Fever" Medline Plus.
12 Gopinath, S., Carden, S., Monach, D. "Shedding light on Salmonella carriers." Trends Microbiology.
13 "Mary Mallon (Typhoid Mary)." American Journal of Public Health.
