Sovaldi and Olysio: Novel Antiviral Treatment for Hepatitis C: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
Hepatitis C is a viral disease that leads to inflammation of the liver, and is part of the Hepatitis virus family, Flaviviridae, of which A and B are the most well known. Chronic hepatitis C virus (HCV) infection leads to scarring of the liver and cirrhosis, which a significantly heightened chance of liver failure and/or liver cancer. While HCV occurs only in human and chimpanzees, it is a highly transmissible virus that occurs through blood contact often in medical, hard labor intensive, sexual intercourse and intravenous drug use settings. Hepatitis C infections are predominantly treated with a medication cocktail consisting of peginterferon and ribavirin, which had a 50% success rate, and would commonly induce multiple side effects such as flu-like symptoms, anemia and depression ( | Hepatitis C is a viral disease that leads to inflammation of the liver, and is part of the Hepatitis virus family, Flaviviridae, of which A and B are the most well known. Chronic hepatitis C virus (HCV) infection leads to scarring of the liver and cirrhosis, which a significantly heightened chance of liver failure and/or liver cancer. While HCV occurs only in human and chimpanzees, it is a highly transmissible virus that occurs through blood contact often in medical, hard labor intensive, sexual intercourse and intravenous drug use settings. Hepatitis C infections are predominantly treated with a medication cocktail consisting of peginterferon and ribavirin, which had a 50% success rate, and would commonly induce multiple side effects such as flu-like symptoms, anemia and depression (Terhune and Brown, 2014). In late 2013, the Federal Drug Administration approved two new non-interferon antiviral drugs to treat Hepatitis C, Gilead's Sovaldi (sofosbuvir) and Johnson and Johnson's Olysio (simeprevir). In test trials involving 197 patients who were not responsive to interferon, the paired administration of Sovaldi and Olysio was shown to clear 90 percent of the Hepatitis C virus (Sheridan, 2014). These two novel drugs will be important in the next few years for curing patients with Hepatitis C, especially if costs can be brought down. | ||
==Section 1== | ==Section 1== | ||
Revision as of 02:18, 11 March 2014
Hepatitis C is a viral disease that leads to inflammation of the liver, and is part of the Hepatitis virus family, Flaviviridae, of which A and B are the most well known. Chronic hepatitis C virus (HCV) infection leads to scarring of the liver and cirrhosis, which a significantly heightened chance of liver failure and/or liver cancer. While HCV occurs only in human and chimpanzees, it is a highly transmissible virus that occurs through blood contact often in medical, hard labor intensive, sexual intercourse and intravenous drug use settings. Hepatitis C infections are predominantly treated with a medication cocktail consisting of peginterferon and ribavirin, which had a 50% success rate, and would commonly induce multiple side effects such as flu-like symptoms, anemia and depression (Terhune and Brown, 2014). In late 2013, the Federal Drug Administration approved two new non-interferon antiviral drugs to treat Hepatitis C, Gilead's Sovaldi (sofosbuvir) and Johnson and Johnson's Olysio (simeprevir). In test trials involving 197 patients who were not responsive to interferon, the paired administration of Sovaldi and Olysio was shown to clear 90 percent of the Hepatitis C virus (Sheridan, 2014). These two novel drugs will be important in the next few years for curing patients with Hepatitis C, especially if costs can be brought down.
Section 1
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: Ebola virus 1.jpeg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
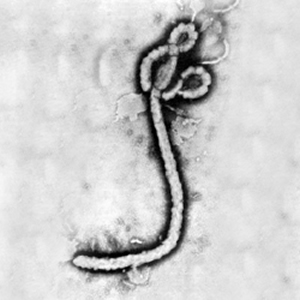
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Overall paper length should be 3,000 words, with at least 3 figures with data.
Section 2
Include some current research in each topic, with at least one figure showing data.
Section 3
Include some current research in each topic, with at least one figure showing data.
Further Reading
[Sample link] Ebola Hemorrhagic Fever—Centers for Disease Control and Prevention, Special Pathogens Branch
References
Edited by (your name here), a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.