The Skin Microbiome and Malaria
Introduction
By Aldis Petriceks
Double brackets: [[
Closed double brackets: ]]
Bold
Italic
Subscript: H2O
Superscript: Fe3+
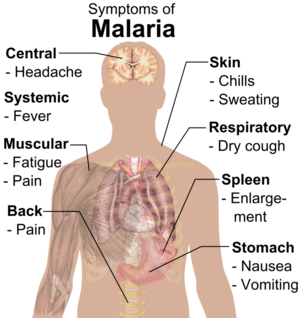
Malaria is a deadly disease caused by the protozoan Plasmodium, and spread through mosquito bites. [1] The most common ailments are fever, chills, and flu-like symptoms which typically begin within a month of infection [2]. Other symptoms include headache, vomiting, jaundice, shivering, and joint pain. The most notable sign of a malaria infection is paroxysm – which includes cycles of cold and shivering followed by fever and sweating. While immediate treatment can typically facilitate a full recovery, those without access to medical care can fall fatal to the disease within days. There are currently 3.4 billion people living in high-risk areas, which led to 500,000 deaths from malaria in 2013 alone (CDC). The majority of these deaths occur in underdeveloped tropical and sub-tropical countries, which in turn incur the bulk of the $12 billion USD yearly cost associated with fighting the disease.
Malaria progresses in two stages. In the first stage, the Plasmodium parasite (in sporozoite form) is injected into the host bloodstream by a mosquito vector, where sporozoites then travel to the liver [3]. Once inside liver cells, these sporozoites spend the next 5-16 days dividing at rapid rates into haploid merozoites. Merozoites build-up in the liver cells causes cell rupture, which then releases the merozoites back into the bloodstream. This begins the second stage of the disease, where the parasites infect red blood cells, undergo many cycles of asexual reproduction, and cause their new host cells to rupture as well, further propagating merozoite production. Infection and rupture cycles repeat every 1-3 days, which manifest themselves in the shivering and fever cycles mentioned above [4].
While the most commonly-known risk factors for malaria are geographic location, socioeconomic level, and lack of proper medical care, research suggests that an individual’s skin microbiome can heavily influence attractiveness to malaria-carrying mosquitoes [5]. Odorous chemical cues are key to mosquito orientation and landing during host location, and skin flora play a large role in the process by affecting human odor production. Specifically, these microbes metabolize lipids and other compounds in naturally odorless sweat, producing volatile odor compounds attractive to anthropophilic mosquitoes [6]. Skin bacteria vary widely in their metabolic pathways, and thus have differing volatile odor productions. As such, composition of skin flora can have large effects on host location in such mosquitoes.
The Skin As An Ecosystem
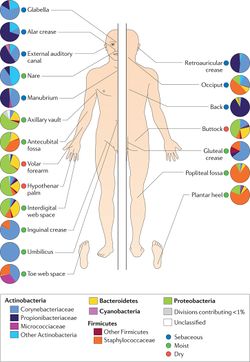
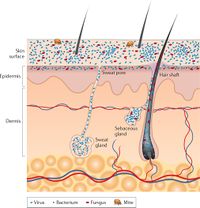
The skin is the largest organ in the body, and constitutes over 1.8 square meters of surface area in which microbes can form habitats [7]. Bacteria, fungi, viruses, and microbial eukaryotes are all common inhabitants of this interface with the outside environment. Similar to any ecosystem, the skin microbiome contains symbiotic organisms of many different natures – many are harmless, others are beneficial to human health, and some are pathogenic and facilitate disease. [8]
The overall environment of the human skin is typically cool in temperature and slightly acidic [9]. However, taking into account the many regions of folds, invaginations, and hair, specific habitats can vary widely between regions of the body. This is especially true in and around the sweat glands. For example, sebaceous glands promote growth of facultative anaerobes such as Propionibacterium acnes due to their oxygen-poor environment [10].
Relative humidity, temperature, and light also varies widely among body regions. Unsurprisingly, these effects can exert great influence on bacterial composition. The skin microbe S. aureus flourishes in the axillary and groin regions, both of which are high humidity and temperature, and low in light exposure [11]. As such, S. aureus is able to grow in high proportions in these environments. On the other hand, most bacterial strains are not able to grow as successfully in dry and open regions such as the arms and legs.
Considering the influence of topography, temperature, humidity, light, sweat gland, and washing, it is difficult to describe a single, overarching microbiome within an individual’s skin. Rather, the skin microbiome can be thought of as several smaller ecosystems, each of which applies its own specific selection pressures. For example, the scalp is high in proportions of sebaceous glands, which strongly selects for facultative anaerobes and lipophiles such as Propionibacterium [12]. This strong selection pressure results in a relatively low species diversity around the sebaceous glands. In moist regions such as the gluteal crease or sole of the foot, Staphylococcus and Corynebacterium tend to dominate, as they are adept at metabolizing the lipids and proteins present in apocrine sweat [13].
While dry, desiccated skin such as that of the arms or legs tends to support less-abundant microbial life than warm, moist regions, these areas provide the greatest levels of bacterial diversity throughout the human skin microbiome. One hypothesis for this phenomenon is that the lack of heat and humidity prevents any single bacterial species from flourishing and thus dominating other species, allowing for smaller proportions of many different bacteria to remain. [14]
Anthropophilic Mosquito Olfaction
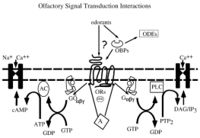
Mosquitoes rely heavily on olfaction during orientation and landing. Olfactory receptors are based in the antennae and maxillary palpi, with each site containing receptors for specific odor compounds [15]. Upon contact with such compounds, olfactory cells send electrical signals to higher-processing centers in the olfactory system [16]. In anthropophilic mosquitoes, these cues typically come in the form of sweat metabolites, which are given their odor by bacteria on the skin epithelium. Studies suggest anthropophilic mosquitoes have evolved to recognize sweat metabolites from human skin bacteria, as these insects greatly prefer natural human scents to synthetic blends of similar salt, water, and heat composition [17]. As variation exists among individuals’ skin flora compositions, relative attractiveness to mosquitoes also differs between humans.
Short-chain carboxylic acids, thioalcohols, benzaldehyde, and other compounds are known to be particularly attractive to anthropophilic mosquitoes [18]. This is because such compounds are common byproducts of volatile-producing skin bacteria, and thus signify nearby presence of a human host. Studies suggest that compounds which are unique to human scent are more attractive to these mosquitoes than more commonly found odor compounds [19].
Specific genes have been found to code for olfaction of human sweat odorants in anthropophilic mosquitoes. For example, the AgOr1 gene codes for mosquito sensory identification of the odorant 4-methylphenol. AgOr2 codes for olfaction of 2-methylphenol, a less-common metabolite of human skin flora It is worth noting that such genes are expressed almost exclusively in female mosquitoes, and transcription of these genes decreases significantly after a blood meal [20].
Regardless of specific olfactory genes, mosquitos can typically detect human odor molecules at short ranges for several weeks after production [21]. Furthermore, anthropophilic species such as the malarial vector Anophiles gambiae Giles sensu stricto prefer human odor compounds over those of other vertebrate species. On the other hand, species such as An. quadriannulatus are preferential towards the scent of cows and other non-human animals.
Microbial Production of Body Odor
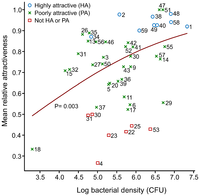
Aerobic coryneforms such as Micrococcaceae and Propionibacterium are the most abundant volatile-producers in high-odor regions such as the underarm [22]. Odor intensity typically increases with coryneform abundance and density, more so than in other skin epithelium bacteria such as Staphylococcus. However, there is still debate as to which bacteria exhibit the strongest association with mosquito attraction.
Attractive bacteria produce the well-known sweat odor by metabolizing lipids and other compounds present in human sweat [23]. Byproducts of this metabolism such as propionic acid, thioalcohols, and benzaldehyde are highly aromatic and give sweat its characteristic, unpleasant smell. Propionic acid, for example, is a product of amino acid breakdown by Propionibacterium. Isovaleric acid is a common metabolic byproduct of the commensal Staphylococcus epidermidis [24].
Because specific volatile production is dependent on the specific metabolic pathways of skin epithelial bacteria, different skin flora compositions can lead to varying odor emanations from the human body. Odor production of individual bacterial strains produce depend largely on the strain’s abundance and capacity to metabolize fatty acids in human sweat. Aerobic coryneforms typically have a high correlation with volatile odor production in humans, owing to their high abundance near sweat glands, and ability to metabolize lipids into free carboxylic acids [25].
Volatile-producing bacteria contain specific genes associated with production of thioalcohols, short-chain carboxylic acids and other volatile compounds. These genes often code for enzymes such as lipases, which metabolize lipids and fatty acids by breaking them down into smaller, more volatile compounds [26]. Interestingly, volatile compound production may vary between growth phases in an individual bacterial species. For example, volatiles produced by skin epithelial bacteria in stationary phase were found to be more than those produced in log phase growth. While a partial explanation for this pertains to the increased bacterial population during stationary phase, analysis also suggests different volatile compounds are produced during different growth phases [27]
Although the large majority of volatiles produced by skin bacteria originate from the metabolism of lipids, breakdown of aliphatic amino acids can also produce malodorous compounds [28]. Volatile compounds produced through this metabolic pathway are typically the same or similar to compounds produced by lipid catabolism. These include short-chain carboxylic acids such as 3-methylbutanoic acid or propanoic acid.
Anthropophilic Mosquito Olfaction

Microbe-Targeted Interventions
References
- ↑ CDC, (2016). "Malaria"
- ↑ Caraballo, H (2014). "Malaria, Dengue, and West Nile Virus"
- ↑ NIAID (2016). "Malaria"
- ↑ NIAID (2016). "Malaria"
- ↑ Carde, H & Gibson, G (2016). "Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues."
- ↑ Olanga et al (2016). "Attraction of Anopheles gambiae to odour baits augmented with heat and moisture."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Rennie et al (1991). "In-vitro and in-vivo studies of human axillary odour and the cutaneous microflora."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ Rennie et al (1991). "In-vitro and in-vivo studies of human axillary odour and the cutaneous microflora."
- ↑ Takken, W & Knols, B (1999). "Odor-mediated behavior of Afrotropical malaria mosquitoes."
- ↑ Zwiebel, L & Takken, W (1999). "Olfactory regulation of mosquito-host interactions."
- ↑ Olanga et al (2010). "Attraction of Anopheles gambiae to odour baits augmented with heat and moisture."
- ↑ Olanga et al (2010). "Attraction of Anopheles gambiae to odour baits augmented with heat and moisture."
- ↑ Hallem et al (2004). "Olfaction: mosquito receptor for human-sweat odorant."
- ↑ Hallem et al (2004). "Olfaction: mosquito receptor for human-sweat odorant."
- ↑ Zwiebel & Takken (2004). "Olfactory regulation of mosquito-host interactions."
- ↑ Rennie et al (1991). "In-vitro and in-vivo studies of human axillary odour and the cutaneous microflora."
- ↑ Lundstrom & Olsson (2010). "Functional neuronal processing of human body odors."
- ↑ Lundstrom & Olsson (2010). "Functional neuronal processing of human body odors."
- ↑ Taylor et al (2003). "Characterization of the microflora of the human axilla."
- ↑ Verhulst et al (2011). "Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes."
- ↑ Verhulstt et al (2011). "Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes."
- ↑ Smallegange et al (2011). "Sweaty skin: an invitation to bite?"
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.