The role of Bifidobacterium on the Immune System: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
[[Image:Bifidobacteriumxtina.png|thumb|300px|right|Picture of Bifidobacterium. From Cann, 2009. ]] | |||
[http://en.wikipedia.org/wiki/Bifidobacterium Bifidobacterium] is a gram-positive lactic acid bacterium and common resident of the human gut microbiome that is speculated to have a huge influence on a range of health-related factors. Due to Bifidobacterium's dominance in infant’s fecal flora and as well as its probiotic application, it has experienced a surge in popularity by the medical and science realm to better understand its mechanistic properties and implications in human health concerns. The mother-to-infant transmission of Bifidobacteria and other bacterial species have been subject to several studies as more knowledge arises on how the initial gut coloinzation can impact an individual’s future health. Because of the speculated health benefits, the applications of Bifidobacterium as a probiotics has become one of the most popularly marketed lactic acid bacteria. | [http://en.wikipedia.org/wiki/Bifidobacterium Bifidobacterium] is a gram-positive lactic acid bacterium and common resident of the human gut microbiome that is speculated to have a huge influence on a range of health-related factors. Due to Bifidobacterium's dominance in infant’s fecal flora and as well as its probiotic application, it has experienced a surge in popularity by the medical and science realm to better understand its mechanistic properties and implications in human health concerns. The mother-to-infant transmission of Bifidobacteria and other bacterial species have been subject to several studies as more knowledge arises on how the initial gut coloinzation can impact an individual’s future health. Because of the speculated health benefits, the applications of Bifidobacterium as a probiotics has become one of the most popularly marketed lactic acid bacteria. | ||
==Transmission of Bifidobacterium== | ==Transmission of Bifidobacterium== | ||
[[Image:Colonization babies.png|thumb|250px|right|The total amount of Bifidobacteria colonization in 17 infants (12 vaginally delivered, 5 cesarean delivered) between days 0 to 7 days old. From Makino, H., Kushiro, A., Ishikawa, E., Kubota, H., Gawad, A., Sakai, T., Oishi, K., Martin, R., Ben-Amor, K., Knol, J., Tanaka, R., 2013. Mother-to-Infant Transmission of Intestinal Bifidobacterial Strains has an Impact on the Early Development of Vaginally Delivered Infant’s Microbiota. PLoS ONE 8(11): e78331. doi:10.1371/journal.pone.0078331 ]] | [[Image:Colonization babies.png|thumb|250px|right|The total amount of Bifidobacteria colonization in 17 infants (12 vaginally delivered, 5 cesarean delivered) between days 0 to 7 days old. From Makino, H., Kushiro, A., Ishikawa, E., Kubota, H., Gawad, A., Sakai, T., Oishi, K., Martin, R., Ben-Amor, K., Knol, J., Tanaka, R., 2013. Mother-to-Infant Transmission of Intestinal Bifidobacterial Strains has an Impact on the Early Development of Vaginally Delivered Infant’s Microbiota. PLoS ONE 8(11): e78331. doi:10.1371/journal.pone.0078331]] | ||
Increasing knowledge of initial gut colonization show a pattern of facultative [http://en.wikipedia.org/wiki/Aerobic_organism aerobes] such as Enterobacteriaceae initially colonizing the microflora, essentially setting the path towards colonization from anaerobic bacterias such as Bifidobacterium longum (Makino, et.al. 2013). This pattern in colonization is interesting in sorts because of its implications of the microbial stimulation that can impact various changes to the development of the immune system. In addition, the transmission of Bifidobacterium and other bacterial species assumes the gastrointestinal tract of newborns to be sterile until birth. | |||
===Vertical Transmission=== | |||
While infants are exposed a wide variety of microbes during birth, only a portion of these microbes colonize the infant’s gut microbiome. Of the microbes that can potentially colonize, the mother’s gut and vaginal microbes are thought to have the greatest influence. When Makino et.al. conducted a study, they concluded vertical transmission of bacterial species including Bifidobacterium was provable as these species were observed in both the mother and infant’s guts after delivery. Thus, the initial exposure when the infant is exposed to the mother’s microbes in the vaginal epithelium and perianal areas is considered to be the primary source of the prominent colonization of Bifidobacterium in the initial gut microbiome. | |||
===Differences Between Birth Mechanisms=== | |||
While vertical transmission of Bifidobacterium colonization occurs through contact during vaginal delivery, Cesarean delivery obstructs a large portion of transmitting the mother’s microbes on the baby’s composition. Because exposure to the mother’s vagina and gut is absent during a Cesarean delivery, transmission of those bacterias to the infant is no longer possible. This mode of delivery is correlated to the large decrease in observable Bifidobacterium colonization in the initial microbiome composition (Biasucci et al., 2010; Makino et al., 2013; Mikami, Kimura, & Takahashi, 2012). Interestingly enough, while initial gut colonization of Bifidobacterium has been shown to be highly variable between vaginally and cesarean delivered infants, colonization patterns to become more cohesive and similar later on. It takes approximately 1 week before any type of bacteria colonizes a sterile gastrointestinal tract, and the fluctuation occurs until approximately 3 months after birth (Björkstén, 2006). | |||
===Decreased, but Still Present=== | |||
While the transmission of Bifidobacterium through the mother’s vaginal and gut composition is eliminated in Cesarean deliveries, other factors can influence the initial colonization pattern of the infant’s gut. The second largest mode speculated to directly influence the colonization is the mode of feeding. Infants can either be breast-fed, formula fed, or fed through a mixture of these two. Feeding greatly influences the transmission, as lactic acids including Bifidobacterium and Lactobacilli are speculated to be present in breast-milk and thus influence the colonization of the infant’s gut (Mikami et al., 2012). In addition, transmission does not necessarily have to be vertical as we observe horizontal transmission patterns. Studies often observe the bacteria species observed in the setting of delivery, for CS infants – the hospital, to hold a large influence in colonizing the initial gut (Mikami et al., 2012). | |||
Revision as of 06:18, 15 April 2014
Bifidobacterium is a gram-positive lactic acid bacterium and common resident of the human gut microbiome that is speculated to have a huge influence on a range of health-related factors. Due to Bifidobacterium's dominance in infant’s fecal flora and as well as its probiotic application, it has experienced a surge in popularity by the medical and science realm to better understand its mechanistic properties and implications in human health concerns. The mother-to-infant transmission of Bifidobacteria and other bacterial species have been subject to several studies as more knowledge arises on how the initial gut coloinzation can impact an individual’s future health. Because of the speculated health benefits, the applications of Bifidobacterium as a probiotics has become one of the most popularly marketed lactic acid bacteria.
Transmission of Bifidobacterium
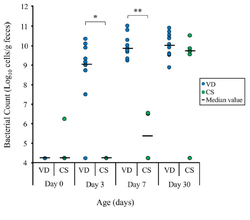
Increasing knowledge of initial gut colonization show a pattern of facultative aerobes such as Enterobacteriaceae initially colonizing the microflora, essentially setting the path towards colonization from anaerobic bacterias such as Bifidobacterium longum (Makino, et.al. 2013). This pattern in colonization is interesting in sorts because of its implications of the microbial stimulation that can impact various changes to the development of the immune system. In addition, the transmission of Bifidobacterium and other bacterial species assumes the gastrointestinal tract of newborns to be sterile until birth.
Vertical Transmission
While infants are exposed a wide variety of microbes during birth, only a portion of these microbes colonize the infant’s gut microbiome. Of the microbes that can potentially colonize, the mother’s gut and vaginal microbes are thought to have the greatest influence. When Makino et.al. conducted a study, they concluded vertical transmission of bacterial species including Bifidobacterium was provable as these species were observed in both the mother and infant’s guts after delivery. Thus, the initial exposure when the infant is exposed to the mother’s microbes in the vaginal epithelium and perianal areas is considered to be the primary source of the prominent colonization of Bifidobacterium in the initial gut microbiome.
Differences Between Birth Mechanisms
While vertical transmission of Bifidobacterium colonization occurs through contact during vaginal delivery, Cesarean delivery obstructs a large portion of transmitting the mother’s microbes on the baby’s composition. Because exposure to the mother’s vagina and gut is absent during a Cesarean delivery, transmission of those bacterias to the infant is no longer possible. This mode of delivery is correlated to the large decrease in observable Bifidobacterium colonization in the initial microbiome composition (Biasucci et al., 2010; Makino et al., 2013; Mikami, Kimura, & Takahashi, 2012). Interestingly enough, while initial gut colonization of Bifidobacterium has been shown to be highly variable between vaginally and cesarean delivered infants, colonization patterns to become more cohesive and similar later on. It takes approximately 1 week before any type of bacteria colonizes a sterile gastrointestinal tract, and the fluctuation occurs until approximately 3 months after birth (Björkstén, 2006).
Decreased, but Still Present
While the transmission of Bifidobacterium through the mother’s vaginal and gut composition is eliminated in Cesarean deliveries, other factors can influence the initial colonization pattern of the infant’s gut. The second largest mode speculated to directly influence the colonization is the mode of feeding. Infants can either be breast-fed, formula fed, or fed through a mixture of these two. Feeding greatly influences the transmission, as lactic acids including Bifidobacterium and Lactobacilli are speculated to be present in breast-milk and thus influence the colonization of the infant’s gut (Mikami et al., 2012). In addition, transmission does not necessarily have to be vertical as we observe horizontal transmission patterns. Studies often observe the bacteria species observed in the setting of delivery, for CS infants – the hospital, to hold a large influence in colonizing the initial gut (Mikami et al., 2012).
Implications towards immunity and other health diseases
While studies on cesarean delivery’s impact on an individual’s immunity have been conducted, further research needs to be engaged to strengthen the relationship. Biasucci et.al. recognized that reduced exposure to a variety of microbes in the environment has been linked to the delay of postnatal maturation of the immune system and thus slower rate to obtaining an optimal balance between TH1 And TH2-like immunity (2010). In addition, naturally born individuals are speculated to have a defense mechanism against Methicillin-resistant Staphylococcus aureus (MRSA) colonization as vaginal microbes temporarily occupy site-specific niches until the eventual community is developed (Dominguez-Bello, et.al., 2010).
Ménard et.al. proved that Bifidobacterium’s ability to stimulate the immune is species specific whereas its influence of the orientation is strain-specific in regards to the balance of T-helper (TH1)/TH2 lymphocyte (2008). The ability to determine the onset of allergic diseases is the mechanism in which T-helper 1 (TH1)/TH2 lymphocyte responds to antigens with a TH2 phenotype (Ménard, et.al. 2008). Bifidobacteria was linked to several different response pathways in the immune system. Dong et.al. conducted their studies based on minimizing and supplementing the presence of Bifidobacteria against a control (2010). Their study proved that the intestinal colonization is able to aid expression of IL-12 in the gut, influence T-cell development in the thymus, and aid the development of Treg response in the gut (Dong et.al., 2010).
Some explain the recent allergy epidemic in the past decades can be explained by CS delivery mechanism, in particular the transmission of Bifidobacterium, as a positive correlation between CS delivery and sensitization to allergy was found in infants with strong family history of allergies. Infants born from mothers with allergies are at a greater risk to obtain an eventual food allergy. An association to subsequent food allergy was at a 4 to 7-fold increase for individuals who were born through cesarean sections from women with various allergies (Eggesbø et.al., 2003). Laubereau, et.al. also conducted a study using hen’s egg as the main IgE allergen marker which proved that infants born through cesarean section were more susceptible to later sensitization (2004).
Uses as a Probiotic
References
Edited by (Christina Kang), a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.