Treatments against Pseudomonas aeruginosa Biofilms in Cystic Fibrosis Patient Lungs
Introduction
Cystic fibrosis (CF), is the most common autosomal recessive genetic disorder of Caucasians in America. Mutations in the CFTR gene cause the chloride channel to malfunction in CF patients, leading to impaired mucociliary clearance of inhaled microbes. Principal mechanisms of lung defense against bacterial colonization include mucociliary clearance, polymorphonuclear neutrophil phagocytosis and local production of antibacterial cationic peptides. However, these defense systems are ineffective against the conditions of increased osmolarity and viscosity in the lungs, and result in chronic lung infection. Pseudomonas aeruginosa, a gram-negative bacterium, thrives in the CF lung environment and is the leading pathogen associated with pulmonary disease in these patients. P. aeruginosa is particularly problematic, infecting the lungs of about 80% of adults with CF. As the bacterial colonies grow, they cause pulmonary problems and, eventually, premature death. The average life expectancy for people with CF is just 37 years. The P. aeruginosa infections survive in the lungs of CF patients due to the adaptive mechanism of biofilm formation. Biofilms are surface attached microbial communities that are surrounded by an extracellular matrix and have great resistance to antibiotics. P. aeruginosa biofilms cause chronic infection because they resist phagocytosis, in addition to other components of the immune system, and have increased tolerance of antibiotic treatments. These bacterial populations are able to adapt to the highly compartmentalized and anatomically deteriorating lung environment of CF patients. Despite rigorous antibiotic and therapeutic therapies, chronic P. aeruginosa lung infections are rarely, if ever, eliminated permanently.
P. Aeruginosa in CF lungs & Biofilm Formation
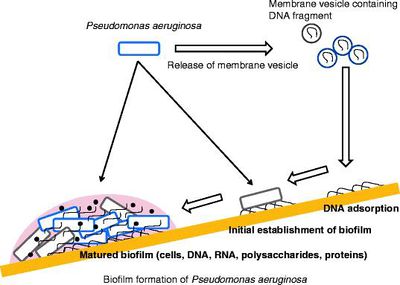
The malfunctioning of CFTR gene causes the body of a CF patient to produce unusually thick and sticky mucus. This mucus builds up and clogs the lungs and pancreas; this obstruction makes breathing very difficult. Copious amounts of sputum are produced in the lungs, which impairs mechanical clearance mechanisms. Bacterial colonization is probably promoted by the unique composition of sputum within the CF lung environment (Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung). CF makes patients susceptible to a broad spectrum of disease phenotypes including failure of lung function and even death. A limited range of microorganisms can colonize the CF lung, and P. aeruginosa in particular is most associated with a decline in lung deterioration and health. Chronic lung infections of P. aeruginosa are the major cause of morbidity and mortality in CF patients [1]. Once formed, these bacterial populations are unmanageable to treatment, mainly due to a biofilm mode of growth. This growth in CF patients’ lungs accompanies an increased frequency of mutations, as well as the adaption of P. aeruginosa to the inflammatory defense mechanism to the lungs and antibiotic treatments. Mechanisms of resistance such as upregulated efflux pumps, mutations of antibiotic target molecules in the bacteria and chromosomal β-lactamase aid P.aeruginosa survival. These conventional resistance mechanisms and formation of mucoid biofilms, biofilms with alginate as a large part of the polysaccharide protection, allow P. aeruginosa to survive for decades despite the antibiotic therapy, while the lung tissue is gradually destroyed. The immense antibiotic resistance may be attributed to low bacterial metabolic activity and increased doubling times of the bacterial cells in CF lungs. Studies indicate that biofilms are a stable point in a biological cycle that includes initiation, maturation, maintenance, and dissolution. The P. aeruginosa appear to initiate biofilm development in response to an unknown environmental cue, possibly nutrient availability [2]
Therapeutic Treatments
Antibiotic Treatment Promise
Antibiotic therapy in CF patients with P. aeruginosa colonized within their lungs typically gives some relief from symptoms but does not cure the persisting infection [D. de Beer, R. Srinivasan, P. S. Stewart, Appl. Environ. Microbiol. 60, 4339 (1994)]. Many researchers see these results as the antibiotics acting upon the planktonic cells that are shed by the biofilms, which alleviate acute symptoms. However, the lung infection persists because antibiotic therapies, thus far, have failed to eliminate the antibiotic resistant sessile biofilm communities.
The sessile communities of P. aeruginosa release antigens while growing in the lung and extremely high concentrations of antibodies to this bacteria are seen circulating in the lung. These antibodies react with their particular antigens in the outer parts of the matrix of the infecting microcolonies. Patients with CF have a high concentration of circulating antibodies to P. aeruginosa, indicating a negative clinical outcome. This has been connected to pulmonary tissue damage that results from the inflammation. Therefore, immune suppression and infection free lungs are not only important, but necessary for beating CF. Recent clinical attempts to prevent the initial colonization of young CF patients by P. aeruginosa with antibiotics are showing promise.
P. aeruginosa’s ability to form antibiotic resistant biofilms is believed to account for the inability of current therapies to eliminate bacterial infections in the lungs of patients with CF. Although the progression of P. aeruginosa colonization makes eradication essentially impossible, early control seems to delay the onset of chronic lung infection. Different antimicrobial treatment protocols have been established once the first sign of P. aeruginosa colonization is exhibited (B. Stuart, J.H. Lin, P.J. Mogayzel. Early eradication of Pseudomonas aeruginosa in patients with cystic fibrosis. Paediatr Respir Rev, 11 (2010), pp. 177–184). The early colonization of these bacteria involves non-mucous colonial morphotypes with low bacterial density. However, once P. aeruginosa begins to colonize, the bacteria density increases and switches to a mucous morphotype with a biofilm mode of growth that is less susceptible to antibiotics (D. Hill, B. Rose, A. Pajkos, M. Robinson, P. Bye, S. Bell et al. Antibiotic susceptibilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J Clin Microbiol, 43 (2005), pp. 5085–5090). Nonetheless, P. aeruginosa may be effectively eradicated during the early stages of colonization with appropriate antibiotic therapy. There is potential prevention of biofilm formation in the CF lung during early stages of colonization. Some evidence exists that suggests early aggressive antibiotic therapy can delay the onset of chronic P. aeruginosa infection in infants or children with CF (Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 2005;4:49–54.). However, reoccurrence or re-infection eventually leads to the development of chronic P. aeruginosa infections by teenagers and adults (Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 2005;4:49–54).
Conclusion
Although there is no antibiotic that completely eradicates the P. aeruginoa biofilms, there may be hope through the use of iron. Iron is vital to many significant physiologic functions in microbial pathogens, such as regulation of gene expression, energy production, and oxygen transport. Iron also promotes biofilm formation on abiotic surfaces, partially regulating surface motility and stabilizing the biofilm polysaccharide matrix. One recent proposed way to eliminate P. aeruginosa biofilm formation is through chelating iron. Compounds like lactoferrin, EDTA, conalbumin and gallium have demonstrated a prevention or disruption of biofilm formation in respiratory epithelial cells. In the presence of low iron conditions induced by an iron chelator, such as lactoferrin, P. aeruginosa forms an irregular biofilm, with a thin layer of cells. In the absence of lactoferrin (and in the presence of adequate iron concentrations) a normal biofilm is formed, comprising a thick layer of cells. Iron levels effect both the formation and maintenance of P. aeruginosa biofilms. A high iron concentration disturbs biofilm formation and stimulates separation of preformed biofilms (Musk et al., 2005; Yang et al., 2007). Musk et al. reported that iron salts (ferric sulfate, ferric chloride, ferrous sulfate and ammonium ferric citrate), at iron concentrations greater than 100 μM inhibited formation of P. aeruginosa biofilms without effecting growth. The first ten hours of development of initial biofilm formation were unaffected; thus the inhibition is not due to decreased adhesion of cells to the surface. More accurately, excess iron seems to disturb advanced stages of biofilm development, so that few cells stick to the surface by 48 hours (Musk et al., 2005)
References
1. M. J. Goldman et al., ibid. 88, 553 (1997) 2. O'Toole, George, and Heidi Kaplan. "Biofilm Formation as Microbial Development." Annual Review of Microbiology 54 (2000): 49-79. Web.