User:S4354350
Veillonella Parvula
Morgan Freney, 43543504, Bench B, 23 September 2016
[1]
Classification
Higher order taxa
Kingdom: Bacteria, Phylum: Firmicute, Class: Negativicutes, Order: Selenomonadales, Family: Veillonellanceae, Genus: Veillonella, Species: Parvula.
In 2010, the genus Veillionella was emended by Marchandin and colleagues who reclassified all 26 genera of the Selenomonas-Megasphaera-Sporomusa group to the new order Selenomonadales in the new class Negativicutes, and divided into two families; Acidaminococcaceae and Veillonellaceae.
Species
Veillonella parvula; Prévot Te3T [ATCC 10790]
Description and significance
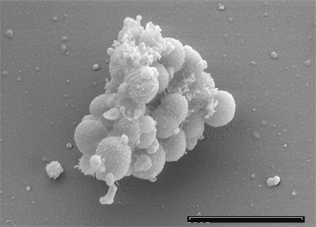
Originally discovered and named as ‘’Staphylococcus parvulus’’ by Veillon and Zuber in 1898, ‘’Veillonella parvula (V. parvula)’’ was later renamed in 1933 by Prévot [1]. A gram-negative species, ‘’V. parvula’’ has unusual characteristics that differs from other closely related species in the Firmicutes phylum, of which the majority are gram positive [1]. A non-motile, non-sporulating, obligate anaerobe, ‘’V. parvula’’ grow as small cocci typically occurring as diplococci, in short chains or in masses. ‘’V. parvula’’ can be identified by its unusual red fluorescence under UV light when grown on trypticase soy agars containing sheep or horse blood. It can also be cultured on multiple other mediums including cysteine-lactose-electrolyte deficient media and hioglycerate broth based on vancomycin selection. ‘’V. parvula’’ is unable to utilise glucose or other sources of carbohydrates, instead reducing nitrate and lactate to produce energy. ‘’V. parvula’’ can be found in the oral cavity, gastrointestinal, respiratory and genito-urinary tract of homoeothermic vertebrates. Part of the commensal oral flora, ‘’V. parvula’’ normally resides in dental plaque. This species can constitute up to 98% of the cultivable ‘’Veillonellae’’ in healthy subgingival sites. It is the only strain of the Veillonellae known to cause oral diseases such as gingivitis. Of significance is the ability of ‘’V. parvula’’ to form a biofilm, where it participates in opportunistic multi-species infections. Only rarely has ‘’V. parvula’’ been isolated in pure culture. It has been implicated in severe infections in diverse sites such as the lungs, sinuses liver, heart, bone and central nervous system.
Genome structure
The genome of strain Te3T consists of a single circular chromosome 2,132,142 base pairs long with a 38.6% GC content. Of the 1,920 genes, 1,859 have been identified as protein-coding, 61 are RNA genes and there are 15 pseudogenes.
Cell structure and metabolism
Gram-negative surface layers consisting of an outer membrane composed of two dense layers 3nm wide separated by a less dense layer of 2nm, a thin peptidoglycan layer, and a cytoplasmic membrane. By negative staining, the outer membrane appears extremely convoluted. Although it is a Gram-negative organism harboring lipopolysaccharide, it is more closely related to Gram-positive species like Sporomusa, Megasphaera or Selenomonas, as they share cadaverine and putrescine in their cell walls. Putrescine or cadaverine links covalently to peptidoglycan and is necessary for normal cell growth.
Well known for its ability to ferment lactate. Veillonella parvula has an optimum growing temperature between 30-37 degrees Celsius and displays a pink to red fluorescence on brain heart infusion agar (BHI) containing either sheep or horse blood. The optimum pH is between 6.5-8.0. The red fluorescence of Veillonellae is due to the production of an atypical catalase containing porphyrin.
Intact Veillonella cells do not utilize carbohydrates for growth, nor are carbohydrates fermented. In cell extracts, there is no detectable glucokinase or fructokinase. Cell extracts do not degrade glucose or fructose unless supplemented with yeast hexokinase. Under these conditions, triose phosphates are formed in the presence of a hydrazine trap. When glucose-C14 plus added hexokinase or fructose-1,6-diphosphate-C14 was incubated with cell extracts, the production of CO2, acetate, pyruvate, propionate, and lactate was detected. It is concluded that, except for a hexokinase, all the activities required for a glycolytic system are present.
Pyruvate, lactate, malate, fumarate, and oxaloacetate are all fermented. Carbohydrates and polyols are not fermented. Veillonellae are characterized by an unusual metabolism using methylmalonyl-CoA decarboxylase to convert the free energy derived from decarboxylation reactions into an electrochemical gradient of sodium ions. Strain Te3T produces propionic and acetic acid, carbon dioxide and hydrogen from lactate and other organic acids like pyruvate, malate or fumarate. V. parvula cannot grow on succinate as a sole carbon source but can decarboxylate succinate during fermentation of lactate or malate, utilising this process for energy conservation.
Ecology
V. parvula is strickly anaerobic and is typically found in the oral cavity, genito-urinary, respiratory, and intestinal tracts of humans and animals. Dental plaque, buccal mucosa, and the tongue are the main ecological niches of V. parvula. Coaggregation of V. parvula with other predominant inhabitants of these niches plays a critical role in the bacterial ecology of the oral cavity by enabling initial attachment and colonisation..
Another characteristic trait of veillonellae is their ability to form intergeneric coaggregates with other bacteria which occur in the same ecological niche. V. parvula utilizes the metabolic end products of co-existing carbohydrate-fermenting bacteria, thereby playing an important role in a natural microbial food chain.
While V. parvula cannot adhere to surfaces itself due to lacking flagella and adhesion structures. The bacterium circumvents this problem by attaching to specific surface structures present on other cells, mediated by lectin-carbohydrate interactions. The coaggregation creates a functional community providing nutrients and protection for all participants.
Pathology
V. parvula is rarely pathogenic in humans. Opportunistic infections caused by V. parvula are most frequently reported as osteomyelitis and endocarditis. Occassionally, cases of bacteraemia are reported.
V. parvula has been suggested to facilitate succession of species in developing oral biofilms in vivo. V. parvula has often been identified in cases of severe early childhood caries, in intraradicular infections including intercranial abscesses, and in dentinal tubules.
The pathogenic roles of Veillonella spp. in oral infections have not yet been fully clarified.
Dual species biofilms containing Veillonella parvula and Streptococcus mutans (S. mutans) have higher resistance to chlorhexidine than either species alone.
V. parvula is susceptible to ampicillin, piperacillin/tazobactam, cefoxitin, cefotetan, ceftriaxone, imipenem, meropenem, clindamycin, bacitracin, metronidazole, remoplanin, trimethoprim/sulfamethoxazole, and vanomycin. It is resistant to amoxycillin and penicillin
When V. parvula is grown with S. mutans resistance against various antimicrobials is enhanced. V. parvula is able to change the gene expression of S. mutans, hence altering its physiology. In this way, V. parvula aids other more pathogenic bacteria such as S. mutans to cause infections such as periodontal disease.
V. parvula also contains two chemically and immunologically distinguishable polysaccharide-lipid complexes which are known virulence factors.
Application to biotechnology
Bioengineering, biotechnologically relevant enzyme/compound production, drug targets,…
Current research
Summarise some of the most recent discoveries regarding this species.
References
References examples
- ↑ MICR3004
This page is written by Morgan Freney for the MICR3004 course, Semester 2, 2016