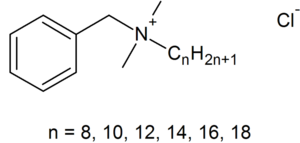User talk:Youngeun92: Difference between revisions
Youngeun92 (talk | contribs) (→General Antiseptic Resistance: new section) |
Youngeun92 (talk | contribs) No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Introduction == | {{Uncurated}} | ||
==Introduction== | |||
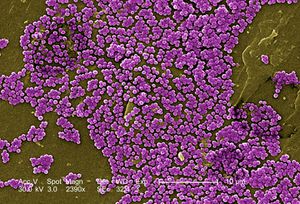
[[File:MRSAs.jpeg|300px|thumb|right|Coloured SEM of methicillin-resistant Staphylococcus aureus]] | |||
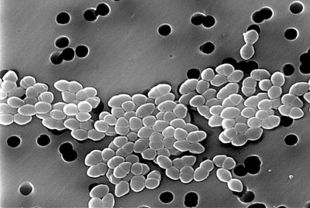
[[File:VREs.jpeg|310px|thumb|right|SEM of vancomycin-resistant Enterococcus ]] | |||
Benzalkonium chloride (BAC) is a major non-alcohol-based active ingredient used for clinical, food line, and domestic household biocides (16,19). A [http://en.wikipedia.org/wiki/Biocide biocide] is a general term for a chemical agent, that may be applied topically in/on living tissue ([http://en.wikipedia.org/wiki/Antiseptic antiseptic]) or on inanimate objects ([http://en.wikipedia.org/wiki/Disinfectant disinfectant]), in order to inhibit growth of ([http://medical-dictionary.thefreedictionary.com/bacteriostatic “-static”]) or kill ([http://www.thefreedictionary.com/Bacteriocidal “-cidal”]) microorganisms (20). | |||
In communities where people are in constant contact with one another and surfaces covered with microorganisms, hand hygiene is important for infection control (16,26,30,31). Studies found the use of antiseptic hand sanitizers subdued the prevalence of the common cold, acute respiratory syndromes, gastroenteritis, viral influenza, and more (1,14,17,24,26,27,31). Similarly, this practice is important in limiting hospital-acquired, or [http://en.wikipedia.org/wiki/Nosocomial_infection nosocomial], infections between patients and clinical staff by limiting spread of opportunists like Pseudomonas aeruginosa, [http://en.wikipedia.org/wiki/Methicillin-resistant_Staphylococcus_aureus methicillin-resistant Staphylococcus aureus] (MRSAs), and [http://en.wikipedia.org/wiki/Vancomycin-resistant_Enterococcus vancomycin-resistant Enterococcus] (VREs) (4,8,12,16,18,29). | |||
In communities where people are in constant contact with one another and surfaces covered with microorganisms, hand hygiene is important for infection control (16,26,30,31). Studies found the use of antiseptic hand sanitizers subdued the prevalence of the common cold, acute respiratory syndromes, gastroenteritis, viral influenza, and more (1,14,17,24,26,27,31). Similarly, this practice is important in limiting hospital-acquired, or nosocomial, infections between patients and clinical staff by limiting spread of opportunists like Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSAs), and vancomycin-resistant Enterococcus (VREs) (4,8,12,16,18,29). | |||
For such preventative measures, there are a variety of hand sanitizers available with alcohol-based and alcohol-free [http://en.wikipedia.org/wiki/Antiseptic#Some_common_antiseptics antiseptic agents] including [http://en.wikipedia.org/wiki/Ethanol#Antiseptic ethanol], [http://en.wikipedia.org/wiki/Triclosan triclosan] and [http://en.wikipedia.org/wiki/Benzalkonium_chloride benzalkonium chloride] (16,20). Recently alcohol-free hand sanitizers with triclosan or benzalkonium chloride have been gaining ground due to concerns that ethanol is dries out the skin, is too toxic, and there are frequent cases of intentional ingestion (9,13,16) Additional concerns involve the extensive use of antiseptics risking the selective survival of antiseptic resistant pathogens, which may be simultaneously selecting for [http://en.wikipedia.org/wiki/Antibiotic_resistance antibiotic resistance] (10,21,25,29). This theory of simultaneous or “cross-selection” suggests that selection of either antiseptic or antibiotic resistance will also select for the other, ultimately resulting in weaker antibacterial therapy and pressure for careful use of both biocides (1,7,25,28). Despite speculations of evolving resistance mechanisms, BACs are extensively used biocides especially efficacious against enveloped microorganisms (16,19,20). | |||
[[File:BAC Towelette.jpeg|200px|thumb|left| A box of benzalkonium chloride-based antiseptic wipes.]] | |||
[[File:X3.gif|320px|thumb|left| A leading benzalkonium chloride-based hand sanitizer with an advertised 99.99% microbe kill rate.]] | |||
[[File:BabyGanics.jpeg|180px|thumb|left| BabyGanics hand sanitizer has a 0.1% benzalkonium chloride content.]] | |||
[[File:Dephyze.jpeg|300px|thumb|left| Dephyze is a benzalkonium-chloride based foaming hand sanitizer brand that claims to have a 99.999% microbe kill rate.]] | |||
[[File:Purell.jpeg|300px|thumb|left| Purell is an ethanol-based antiseptic hand sanitizer advertised to kill 99.99% of hand microbes within 15 seconds.]] | |||
==General Mode of Antiseptic Activity== | |||
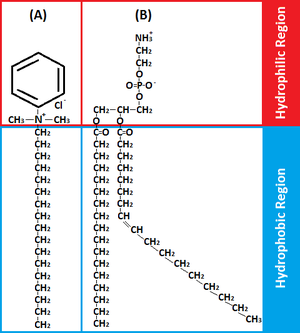
[[File:BAC-Phospholipid.png|300px|thumb|right| Comparative amphiphilicity of benzalkonium chloride and phospholipid molecules.]] | |||
Since the discovery of [http://en.wikipedia.org/wiki/Penicillin penicillin], research of [http://en.wikipedia.org/wiki/Antibiotic_resistance#Mechanisms antibiotic mechanisms] garnered much attention with widespread studies dissecting details of resistance against [http://en.wikipedia.org/wiki/Vancomycin vancomycin], [http://en.wikipedia.org/wiki/Beta-lactam β-lactams], and other antibiotics (12,15,32). In contrast, studies on the mechanistic details of particular antiseptics remains largely inconclusive aside from the generality that antiseptics have less specific activities at multiple targets compared to antibiotics (18, 20). This broad level of activity is well summarized by McDonnell and Russell (1999), as the antiseptic first interacting with the cell surface, then penetrating into the cell, and finally acting at intracellular target sites. Sheldon (2005) further suggests that the details behind each antiseptic depend on the biocide’s chemical nature, the pathogen, and the test conditions (including but not limited to antiseptic concentration, pH, time of exposure, and temperature). In this way, antiseptic mechanisms lack mechanistic details, but the general activity is still well agreed upon. | |||
==Quaternary Ammonium Compounds (QACs)== | |||
[[File:BAC.png|300px|thumb|left| Benzalkonium chloride.]] | |||
BACs are classified as [http://en.wikipedia.org/wiki/Category:Quaternary_ammonium_compounds Quaternary Ammonium Compounds], which are positively charged derivatives of ammonium compounds with the chemical formula NR4+, where R can be different carbon-hydrogen containing groups (23). QACs are commonly used as antiseptic agents because of their cationic [http://en.wikipedia.org/wiki/Amphiphile amphiphilic] property, having a distinct hydrophobic and hydrophilic region, resulting from [http://en.wikipedia.org/wiki/Nucleophilic_substitution nucleophilic substitution] of alkyldimethylamine and benzyl chloride (10). BAC’s hydrophilic cationic region destabilizes the pathogen’s surface by forming electrostatic interactions with negatively charged components (10,20,32). These interactions effectively outcompete the divalent cations, which normally stabilizes surface structures by linking adjacent negatively-charged components (10,20,32). Once close contact is accomplished by the hydrophilic region, the BAC’s hydrophobic region proceeds to penetrate the hydrophobic bilayer to cause cell leakage and lysis (10,19,20). The ultimate effect of BACs is to damage the pathogen’s membrane, thus for bacteria, disrupting essential cell processes like [http://en.wikipedia.org/wiki/ATP_synthase#Physiological_role ATP synthesis] or solute uptake (20). Therefore BAC’s amphiphilicity is critical in interacting with and perturbing target membranes for efficacious antimicrobial action. | |||
==Susceptible Microorganisms== | |||
The key component of BAC’s antimicrobial activity is membrane destruction, which is most effective against Gram-positive bacteria, some Gram-negative bacteria, some enveloped viruses, fungi, yeasts and protozoa (10). | The key component of BAC’s antimicrobial activity is membrane destruction, which is most effective against Gram-positive bacteria, some Gram-negative bacteria, some enveloped viruses, fungi, yeasts and protozoa (10). | ||
b)Enveloped viruses | ===Non-sporulating Gram-positive bacteria=== | ||
Some viruses require a lipid envelope, which serves a dual function: first, as a protective barrier from harsh environmental conditions of pH or desiccation, and secondly as an undesirable target for BAC-based antiseptics (20). Therefore, enveloped viruses like HIV, HBV, influenza, measles, vaccinia, meningopneumonitis, | [[File:3D image of a measles virus.jpeg|230px|thumb|right|A three-dimensional image of the surface of an enveloped measles virus.]] | ||
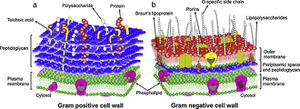
[[File:Cell_wall.jpeg|300px|thumb|left|General comparison of Gram-positive (a) and Gram-negative (b) bacterial cell wall structure.]][http://en.wikipedia.org/wiki/Staphylococcus_aureus Staphylococcus aureus] is a Gram-positive bacterium that can survive on antiseptic-free hands for at least 150 minutes, which is enough time for it to spread and persist as a leading cause of nosocomial infections (16). However, S. aureus is readily killed with BACs because their cell walls are chiefly composed of slightly-negatively-charged [http://en.wikipedia.org/wiki/Peptidoglycan peptidoglycan] and [http://en.wikipedia.org/wiki/Teichoic_acid techoic acid]s (10,20). These surface structures also lack effective permeability properties and allow uptake of more BACs and other antimicrobial substances (10,20). | |||
===Enveloped viruses=== | |||
Some viruses require a lipid envelope, which serves a dual function: first, as a protective barrier from harsh environmental conditions of pH or desiccation, and secondly as an undesirable target for BAC-based antiseptics (20). Therefore, enveloped viruses like [http://en.wikipedia.org/wiki/HIV human immunodeficiency virus] (HIV), [http://en.wikipedia.org/wiki/Hepatitis_B#Virology hepatitis B virus] (HBV), [http://en.wikipedia.org/wiki/Influenza#Virology influenza virus], [http://en.wikipedia.org/wiki/Measles measles virus], [http://en.wikipedia.org/wiki/Vaccinia vaccinia virus], meningopneumonitis virus, [http://en.wikipedia.org/wiki/Semliki_Forest_virus Semliki Forest virus], [http://en.wikipedia.org/wiki/Canine_distemper canine distemper virus], [http://en.wikipedia.org/wiki/Rabies_virus rabies virus], [http://ultimatefowl.atwiki.com/page/Infectious%20Laryngotracheitis fowl laryngotracheitis], and feline pneumonitis virus are all susceptible to BACs (2,20,24). | |||
==General Antiseptic Resistance== | |||
Despite suggestions that the non-specificity and multiple target range of antiseptic mechanisms minimizes selective pressure for resistant species, it is generally thought that widespread use has indeed selected for antiseptic resistance as found with antibiotics (12,20,29). Currently suggested antiseptic resistance mechanisms uncannily resemble those conferring antibiotic resistance: enzymatic degradation of antiseptic agents, [http://en.wikipedia.org/wiki/Biofilm biofilm] formations, active [http://en.wikipedia.org/wiki/Efflux_(microbiology) efflux], and impermeability (29,32). Generally, these mechanisms may originate as either an intrinsic (natural) or acquired property (by genetic [http://en.wikipedia.org/wiki/Mutation mutation] or [http://en.wikipedia.org/wiki/Horizontal_gene_transfer horizontal gene transfer]) of the microorganism (20). In the case of rapidly growing microorganisms, beneficial mutations are selected for then stabilized in a growing population resulting in acquired resistance (20,22). Additionally, studies are finding that genes encoding resistance components located on [http://en.wikipedia.org/wiki/Mobile_genetic_elements mobile genetic elements] (eg. [http://en.wikipedia.org/wiki/Mobile_genetic_elements plasmid]s or [http://en.wikipedia.org/wiki/Transposable_element transposon]s) may be transferred from one microorganism to another, as is the case with Staphylococci carrying QAC-resistant plasmids encoding efflux pumps (3,7,25). Therefore in response to antiseptic action, different microorganisms exhibit these aforementioned intrinsic and acquired antiseptic resistance mechanisms. | |||
==Impermeability of Benzalkonium Chloride== | |||
[[File:SEM pseudomonas aeruginosa.jpeg|260px|thumb|right|(Scanning Electron Microscopy image of Pseudomonas aeruginosa.]] | |||
In particular, impermeability by additional or architecturally-complex surface layers in addition to the cellular membrane are commonly exhibited by a variety of microorganisms that render BAC less efficacious (18,20). Not only is BAC prevented from causing cell lysis, but the uptake of BAC and other active agents in hand sanitizers is severely inhibited (20). Therefore, even at high concentrations, BACs tend to be “non-cidal” and are rather [http://medical-dictionary.thefreedictionary.com/bacteriostatic bacteriostatic], mycobacteriostatic, and sporostatic (20). | |||
===Bacteriostatic=== | |||
[http://en.wikipedia.org/wiki/Pseudomonas_aeruginosa Pseudomonas aeruginosa] is a Gram-negative bacterium with reduced sensitivity to BACs, and is of clinical importance as a common [http://en.wikipedia.org/wiki/Opportunistic_infection#Types_of_infections opportunistic pathogen] causing nosocomial respiratory tract infections like [http://en.wikipedia.org/wiki/Pneumonia pneumonia] (5,6,20,). Generally, Gram-negative bacteria like P. aeruginosa are less permeable to QACs than non-sporulating, non-mycobacterial Gram-positive organisms (6,20). This marked difference in uptake regulation by Gram-negative bacteria is mediated by structural differences including an [http://en.wikipedia.org/wiki/Bacterial_outer_membrane outer membrane], [http://en.wikipedia.org/wiki/Porin_(protein) porin]s, and [http://en.wikipedia.org/wiki/Efflux_(microbiology)#Bacterial_efflux_pumps efflux pump]s (16,29). While inner membranes have a conventional phospholipid bilayer, the outer leaflet of an outer membrane is composed of [http://en.wikipedia.org/wiki/Lipopolysaccharide lipopolysaccharide]s (LPS) (32). The outer membrane’s reduced permeability is a result of strong LPS-LPS lateral interactions, where a highly anionic “R-core” region on the LPS links interacts with soluble cations to link LPS molecules together (see Fig#) (16,29,32). The outer membrane’s hydrophobic core has greater [http://en.wikipedia.org/wiki/London_dispersion_force London disperson forces] as LPS has up to seven hydrocarbon tails per molecule compared to two per each phospholipid molecule of the inner membrane (32). Small porins studding this outer membrane facilitates diffusion of hydrophilic solutes, but can strictly restrict larger molecules of BAC (16,29). Additionally, efflux proteins in the inner membrane may actively pump out BACs that make it past the outer membrane (mechanism not covered in this wiki, see "[http://en.wikipedia.org/wiki/Efflux_(microbiology)#Bacterial_efflux_pumps efflux pump]" for details) (16,29). Although P. aeruginosa is only a model organism and there have been reports of BAC efficaciously destructing the outer membranes of other Gram-negative species, P. aeruginosa maximizes BAC impermeability as an antiseptic-resistance measure (19). | |||
[[File:Mycobacterial_cell_wall.png|450px|thumb|right|Schematic diagram of Mycobacterial cell wall structure.]] | |||
===Mycobacteriostatic=== | |||
[http://en.wikipedia.org/wiki/Mycobacterium Mycobacteria] are some of the most antiseptic-resistant opportunistic pathogens, characterized by highly hydrophobic cell walls of a complex [http://en.wikipedia.org/wiki/Arabinogalactan mycoyl arabinogalactan-peptidoglycan] skeletal architecture (11,18). Although the specific component conferring antiseptic resistance remains undecided, experiments involving cell wall synthesis inhibitors suggests that a cell wall component like lipid content is responsible (29). Further studies supports the idea of lipid content determining BAC (im)permeability, as species with high lipid content like [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis] were found to be less antiseptic sensitive than [http://en.wikipedia.org/wiki/Mycobacterium_phlei Mycobacterium phlei] with lower lipid content (29,20). | |||
[[File:Clostridium_difficile.jpeg|400px|thumb|left|(Clostridium difficile.]] | |||
===Sporostatic=== | |||
[http://en.wikipedia.org/wiki/Clostridium_difficile Clostridium difficile] is a major spore forming bacteria, causing 15-55% of all nosocomial diarrheas (16,18,20). Similar to Gram-negative and myco- bacteria, additional impermeability layers of complex [http://en.wikipedia.org/wiki/Endospore#Structure spore coat(s)] hinders the permeability and activity of BACs (20). | |||
==References== | |||
1.Aiello, A.E., Coulborn, R.M., Perez, V., and Larson, E.L. 2008. Effect of hand hygiene on infectious disease risk in the community setting. American Journal of Public Health, 98 (8): 1372-1381. | |||
2.Armstrong, J.A., and Froelich, E.J. 1964. Inactivation of viruses by benzalkonium chloride. Applied Microbiology, 12 (2): 132-137. | |||
3.Bjorland, J., Steinum, T., Kvitle, B., Waage, S., Sunde, M., and Heir, E. 2005. Widespread distribution of disinfectant resistance genes among Staphylococci of bovine and caprine origin in Norway. Journal of Clinical Microbiology, 43 (9): 4363-4369. | |||
4.Campanac, C., Pineau, L., Payard, A., Baziard-Mouysset, G., and Roques, C. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrobial agents and chemotherapy, 46 (5): 1469-1474. | |||
5.Chi, S.Y., Kim, T.O., Park, C.W., Yu, J.Y., Lee, B., Lee, H.S., Kim, Y.I., Lim, S.C., and Kwon, Y.S. 2012. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberculosis and Respiratory Diseases, 73 (1): 32-37. | |||
6.Chuanchuen, R., Beinlich, K., Hoang, T.T., Becher, A., Karkhoff-Schweizer, R.R., and Schweizer, H.P. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrobial Agents and Chemotherapy, 45 (2): 428-432. | |||
7.Ciric, L., Mullany, P., and Roberts, A.P. 2011. Antibiotic and antiseptic resistance genes are linked on a novel mobile genetic element: Tn6087. Journal of Antimicrobial Chemotherapy, 66: 2235-2239. | |||
8.Cook, H.A., Cimiotti, J.P., Phyllis D.L., Saiman, L., and Larson, E.L. 2007. Antimicrobial resistance patterns of colonizing flora on nurses’ hands in the neonatal intensive care unit. American Journal of Infection Control, 35 (4): 231-236. | |||
9.Engel, J.S., and Spiller, H.A. 2010. Acute ethanol poisoning in a 4-year-old as a result of ethanol-based hand-sanitizer ingestion. Pediatric Emergency Care, 26 (70): 508-509. | |||
10.Fazlara, A., and Ekhtelat, M. 2012. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. American-Eurasian Journal of Agricultural & Environmental Science, 12 (1): 23-29. | |||
11.Frenzel, E., Schmidt, S., Niederweis, M., and Steinhauer, K. 2011. Importance of porins for biocide efficacy against Mycobacterium smegmatis. Applied and Environmental Microbiology, 77 (9): 3068-3073. | |||
12.Gordin, F.M., Schultz, M.E., Huber, R.A., and Gill, J.A. 2005. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infection control and hospital epidemiology, 26 (7): 650-653. | |||
13.Gormley, N.J., Bronstein, A.C., Rasimas, J.J., Pao, M., Wratney, A.T., Sun, J., Austin, H.A., and Suffredini, A.F. 2012. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Critical Care Medicine, 40 (1): 290-294. | |||
14.Hammond, B., Ali, Y., Fendler, E., Dolan, M,D.S. 2000. Effect of hand sanitizer use on elementary school absenteeism. American Journal of Infection Control, 28:340–6. | |||
15.Hwaser, S. 2012. Surveillance programmes and antibiotic resistance: worldwide and regional monitoring of antibiotic resistance trends. Handbook of Experimental Pharmacology, 211: 31-43. | |||
16.Kampf, G., and Kramer, A. 2004. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17 (4): 863-893. | |||
17.Lau, C.H., Springston, E.E. , Sohn, M.W., Mason, I., Gadola, E., Damitza, M., and Gupta, R.S. 2012. Hand hygiene instruction decreases illness-related absenteeism in elementary schools: a prospective cohort study. Biomedical Central Pediatrics, 12 (52): 1-7. | |||
18.Leaper, D., McBainm, A.J., Kramer, A., Assdian, O., Sanchez, J.L.A., Lumio, J, and Kiernan, M. 2010. Healthcare associated infection: novel strategies and antimicrobial implants to prevent surgical site infection. Anals of the Royal College of Surgeons of England, 92: 452-458. | |||
19.Mangalappalli-Illathu, A.K. , and Korber, D.R. 2006. Adaptive resistance and differential protein expression on Salmonella enteric serovar enteritidis biofilms exposed to benzalkonium chloride. Antimicrobial agents and chemotherapy, 50 (11): 3588-3596. | |||
20.McDonnell, G., and Russell, A.D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews, 12 (1): 147-179. | |||
21.Moen, B., Rudi, K., Bore, E., and Langsrud, S. 2012. Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of E. coli with inheritable characteristics. International Journal of Molecular Sciences, 12: 4101-4123. | |||
22.Nasvall, J., Sun, L., Roth, J.R., and Andersson, D.I. 2012. Real-time evolution of new genes by innovation, amplification and divergence. Science, 338 (6105):384-387. | |||
23.Nic, M., Jirat, B., and Kosata, B. 2006. IUPAC of quaternary ammonium compounds [online]. Available from http://goldbook.iupac.org/Q05003.html [accessed 26 October 2012]. | |||
24.Oxford, J.S., Potter, W., McLaren, C., and Hardy, W. 1971. Inactivation of influenza and other viruses by a mixture of virucidal compounds. American Society for Microbiology, 21 (4): 606-610. | |||
25.Pagear, A., Singh, J., and Batish, V.K. 2011. Efflux mediated adaptive and cross resistance to ciprofloxacin and benzalkonium chloride in Pseudomonas aeruginosa of dairy origin. Journal of Basic Microbiology, 51: 289-295. | |||
26.Prazuck, T., Compte-Nguyen, G., Pelat, C., Sunder, S., and Blanchon, T. 2010. Reducing gastroenteritis occurrences and their consequences in elementary schools with alcohol-based hand sanitizers. The Pediatric Infectious Disease Journal, 29 (11): 994-998. | |||
27.Reynolds, S.A., Levy, F., and Walker, E.S. 2006. Hand sanitizer alert. Emerging Infectious Diseases, 12 (3): 527-529. | |||
28.Sidhu, M.S., Heir, E., Sorum, and Holck A. 2001. Genetic linkage between resistance to quaternary ammonium compounds and beta-lactam antibiotics in food-related Staphylococcus spp. Microbial Drug Resistance, 7 (4): 363-371. | |||
29.Sheldon, A.T. 2005. Antiseptic “resistance”: real or perceived threat? Clinical Infectious Diseases, 40 (11): 1650-1656. | |||
30.To, M.S., Favrin, S., Romanova, N., and Griffiths, M.W. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Applied and Environmental Microbiology, 68 (11): 5258-5264. | |||
31.White, C., Kolble, R., Carlson, R., Lipson, N., Dolan, M., Ali, Y., and Cline, M. 2003. The effect of hand hygiene on illness rate among students in university residence halls. American Journal of Infection Control, 31:364–370. | |||
32.MICB 201 textbook citation?????? | |||
<!-- Do not edit or remove this line -->[[Category:Pages edited by students of Angela Kent at the University of Illinois at Urbana-Champaign]] | |||
Latest revision as of 01:50, 19 November 2012
Introduction
Benzalkonium chloride (BAC) is a major non-alcohol-based active ingredient used for clinical, food line, and domestic household biocides (16,19). A biocide is a general term for a chemical agent, that may be applied topically in/on living tissue (antiseptic) or on inanimate objects (disinfectant), in order to inhibit growth of (“-static”) or kill (“-cidal”) microorganisms (20).
In communities where people are in constant contact with one another and surfaces covered with microorganisms, hand hygiene is important for infection control (16,26,30,31). Studies found the use of antiseptic hand sanitizers subdued the prevalence of the common cold, acute respiratory syndromes, gastroenteritis, viral influenza, and more (1,14,17,24,26,27,31). Similarly, this practice is important in limiting hospital-acquired, or nosocomial, infections between patients and clinical staff by limiting spread of opportunists like Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSAs), and vancomycin-resistant Enterococcus (VREs) (4,8,12,16,18,29).
For such preventative measures, there are a variety of hand sanitizers available with alcohol-based and alcohol-free antiseptic agents including ethanol, triclosan and benzalkonium chloride (16,20). Recently alcohol-free hand sanitizers with triclosan or benzalkonium chloride have been gaining ground due to concerns that ethanol is dries out the skin, is too toxic, and there are frequent cases of intentional ingestion (9,13,16) Additional concerns involve the extensive use of antiseptics risking the selective survival of antiseptic resistant pathogens, which may be simultaneously selecting for antibiotic resistance (10,21,25,29). This theory of simultaneous or “cross-selection” suggests that selection of either antiseptic or antibiotic resistance will also select for the other, ultimately resulting in weaker antibacterial therapy and pressure for careful use of both biocides (1,7,25,28). Despite speculations of evolving resistance mechanisms, BACs are extensively used biocides especially efficacious against enveloped microorganisms (16,19,20).
General Mode of Antiseptic Activity
Since the discovery of penicillin, research of antibiotic mechanisms garnered much attention with widespread studies dissecting details of resistance against vancomycin, β-lactams, and other antibiotics (12,15,32). In contrast, studies on the mechanistic details of particular antiseptics remains largely inconclusive aside from the generality that antiseptics have less specific activities at multiple targets compared to antibiotics (18, 20). This broad level of activity is well summarized by McDonnell and Russell (1999), as the antiseptic first interacting with the cell surface, then penetrating into the cell, and finally acting at intracellular target sites. Sheldon (2005) further suggests that the details behind each antiseptic depend on the biocide’s chemical nature, the pathogen, and the test conditions (including but not limited to antiseptic concentration, pH, time of exposure, and temperature). In this way, antiseptic mechanisms lack mechanistic details, but the general activity is still well agreed upon.
Quaternary Ammonium Compounds (QACs)
BACs are classified as Quaternary Ammonium Compounds, which are positively charged derivatives of ammonium compounds with the chemical formula NR4+, where R can be different carbon-hydrogen containing groups (23). QACs are commonly used as antiseptic agents because of their cationic amphiphilic property, having a distinct hydrophobic and hydrophilic region, resulting from nucleophilic substitution of alkyldimethylamine and benzyl chloride (10). BAC’s hydrophilic cationic region destabilizes the pathogen’s surface by forming electrostatic interactions with negatively charged components (10,20,32). These interactions effectively outcompete the divalent cations, which normally stabilizes surface structures by linking adjacent negatively-charged components (10,20,32). Once close contact is accomplished by the hydrophilic region, the BAC’s hydrophobic region proceeds to penetrate the hydrophobic bilayer to cause cell leakage and lysis (10,19,20). The ultimate effect of BACs is to damage the pathogen’s membrane, thus for bacteria, disrupting essential cell processes like ATP synthesis or solute uptake (20). Therefore BAC’s amphiphilicity is critical in interacting with and perturbing target membranes for efficacious antimicrobial action.
Susceptible Microorganisms
The key component of BAC’s antimicrobial activity is membrane destruction, which is most effective against Gram-positive bacteria, some Gram-negative bacteria, some enveloped viruses, fungi, yeasts and protozoa (10).
Non-sporulating Gram-positive bacteria
Staphylococcus aureus is a Gram-positive bacterium that can survive on antiseptic-free hands for at least 150 minutes, which is enough time for it to spread and persist as a leading cause of nosocomial infections (16). However, S. aureus is readily killed with BACs because their cell walls are chiefly composed of slightly-negatively-charged peptidoglycan and techoic acids (10,20). These surface structures also lack effective permeability properties and allow uptake of more BACs and other antimicrobial substances (10,20).
Enveloped viruses
Some viruses require a lipid envelope, which serves a dual function: first, as a protective barrier from harsh environmental conditions of pH or desiccation, and secondly as an undesirable target for BAC-based antiseptics (20). Therefore, enveloped viruses like human immunodeficiency virus (HIV), hepatitis B virus (HBV), influenza virus, measles virus, vaccinia virus, meningopneumonitis virus, Semliki Forest virus, canine distemper virus, rabies virus, fowl laryngotracheitis, and feline pneumonitis virus are all susceptible to BACs (2,20,24).
General Antiseptic Resistance
Despite suggestions that the non-specificity and multiple target range of antiseptic mechanisms minimizes selective pressure for resistant species, it is generally thought that widespread use has indeed selected for antiseptic resistance as found with antibiotics (12,20,29). Currently suggested antiseptic resistance mechanisms uncannily resemble those conferring antibiotic resistance: enzymatic degradation of antiseptic agents, biofilm formations, active efflux, and impermeability (29,32). Generally, these mechanisms may originate as either an intrinsic (natural) or acquired property (by genetic mutation or horizontal gene transfer) of the microorganism (20). In the case of rapidly growing microorganisms, beneficial mutations are selected for then stabilized in a growing population resulting in acquired resistance (20,22). Additionally, studies are finding that genes encoding resistance components located on mobile genetic elements (eg. plasmids or transposons) may be transferred from one microorganism to another, as is the case with Staphylococci carrying QAC-resistant plasmids encoding efflux pumps (3,7,25). Therefore in response to antiseptic action, different microorganisms exhibit these aforementioned intrinsic and acquired antiseptic resistance mechanisms.
Impermeability of Benzalkonium Chloride
In particular, impermeability by additional or architecturally-complex surface layers in addition to the cellular membrane are commonly exhibited by a variety of microorganisms that render BAC less efficacious (18,20). Not only is BAC prevented from causing cell lysis, but the uptake of BAC and other active agents in hand sanitizers is severely inhibited (20). Therefore, even at high concentrations, BACs tend to be “non-cidal” and are rather bacteriostatic, mycobacteriostatic, and sporostatic (20).
Bacteriostatic
Pseudomonas aeruginosa is a Gram-negative bacterium with reduced sensitivity to BACs, and is of clinical importance as a common opportunistic pathogen causing nosocomial respiratory tract infections like pneumonia (5,6,20,). Generally, Gram-negative bacteria like P. aeruginosa are less permeable to QACs than non-sporulating, non-mycobacterial Gram-positive organisms (6,20). This marked difference in uptake regulation by Gram-negative bacteria is mediated by structural differences including an outer membrane, porins, and efflux pumps (16,29). While inner membranes have a conventional phospholipid bilayer, the outer leaflet of an outer membrane is composed of lipopolysaccharides (LPS) (32). The outer membrane’s reduced permeability is a result of strong LPS-LPS lateral interactions, where a highly anionic “R-core” region on the LPS links interacts with soluble cations to link LPS molecules together (see Fig#) (16,29,32). The outer membrane’s hydrophobic core has greater London disperson forces as LPS has up to seven hydrocarbon tails per molecule compared to two per each phospholipid molecule of the inner membrane (32). Small porins studding this outer membrane facilitates diffusion of hydrophilic solutes, but can strictly restrict larger molecules of BAC (16,29). Additionally, efflux proteins in the inner membrane may actively pump out BACs that make it past the outer membrane (mechanism not covered in this wiki, see "efflux pump" for details) (16,29). Although P. aeruginosa is only a model organism and there have been reports of BAC efficaciously destructing the outer membranes of other Gram-negative species, P. aeruginosa maximizes BAC impermeability as an antiseptic-resistance measure (19).
Mycobacteriostatic
Mycobacteria are some of the most antiseptic-resistant opportunistic pathogens, characterized by highly hydrophobic cell walls of a complex mycoyl arabinogalactan-peptidoglycan skeletal architecture (11,18). Although the specific component conferring antiseptic resistance remains undecided, experiments involving cell wall synthesis inhibitors suggests that a cell wall component like lipid content is responsible (29). Further studies supports the idea of lipid content determining BAC (im)permeability, as species with high lipid content like Mycobacterium tuberculosis were found to be less antiseptic sensitive than Mycobacterium phlei with lower lipid content (29,20).
Sporostatic
Clostridium difficile is a major spore forming bacteria, causing 15-55% of all nosocomial diarrheas (16,18,20). Similar to Gram-negative and myco- bacteria, additional impermeability layers of complex spore coat(s) hinders the permeability and activity of BACs (20).
References
1.Aiello, A.E., Coulborn, R.M., Perez, V., and Larson, E.L. 2008. Effect of hand hygiene on infectious disease risk in the community setting. American Journal of Public Health, 98 (8): 1372-1381.
2.Armstrong, J.A., and Froelich, E.J. 1964. Inactivation of viruses by benzalkonium chloride. Applied Microbiology, 12 (2): 132-137.
3.Bjorland, J., Steinum, T., Kvitle, B., Waage, S., Sunde, M., and Heir, E. 2005. Widespread distribution of disinfectant resistance genes among Staphylococci of bovine and caprine origin in Norway. Journal of Clinical Microbiology, 43 (9): 4363-4369.
4.Campanac, C., Pineau, L., Payard, A., Baziard-Mouysset, G., and Roques, C. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrobial agents and chemotherapy, 46 (5): 1469-1474.
5.Chi, S.Y., Kim, T.O., Park, C.W., Yu, J.Y., Lee, B., Lee, H.S., Kim, Y.I., Lim, S.C., and Kwon, Y.S. 2012. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberculosis and Respiratory Diseases, 73 (1): 32-37.
6.Chuanchuen, R., Beinlich, K., Hoang, T.T., Becher, A., Karkhoff-Schweizer, R.R., and Schweizer, H.P. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrobial Agents and Chemotherapy, 45 (2): 428-432.
7.Ciric, L., Mullany, P., and Roberts, A.P. 2011. Antibiotic and antiseptic resistance genes are linked on a novel mobile genetic element: Tn6087. Journal of Antimicrobial Chemotherapy, 66: 2235-2239.
8.Cook, H.A., Cimiotti, J.P., Phyllis D.L., Saiman, L., and Larson, E.L. 2007. Antimicrobial resistance patterns of colonizing flora on nurses’ hands in the neonatal intensive care unit. American Journal of Infection Control, 35 (4): 231-236.
9.Engel, J.S., and Spiller, H.A. 2010. Acute ethanol poisoning in a 4-year-old as a result of ethanol-based hand-sanitizer ingestion. Pediatric Emergency Care, 26 (70): 508-509.
10.Fazlara, A., and Ekhtelat, M. 2012. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. American-Eurasian Journal of Agricultural & Environmental Science, 12 (1): 23-29.
11.Frenzel, E., Schmidt, S., Niederweis, M., and Steinhauer, K. 2011. Importance of porins for biocide efficacy against Mycobacterium smegmatis. Applied and Environmental Microbiology, 77 (9): 3068-3073.
12.Gordin, F.M., Schultz, M.E., Huber, R.A., and Gill, J.A. 2005. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infection control and hospital epidemiology, 26 (7): 650-653.
13.Gormley, N.J., Bronstein, A.C., Rasimas, J.J., Pao, M., Wratney, A.T., Sun, J., Austin, H.A., and Suffredini, A.F. 2012. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Critical Care Medicine, 40 (1): 290-294.
14.Hammond, B., Ali, Y., Fendler, E., Dolan, M,D.S. 2000. Effect of hand sanitizer use on elementary school absenteeism. American Journal of Infection Control, 28:340–6.
15.Hwaser, S. 2012. Surveillance programmes and antibiotic resistance: worldwide and regional monitoring of antibiotic resistance trends. Handbook of Experimental Pharmacology, 211: 31-43.
16.Kampf, G., and Kramer, A. 2004. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17 (4): 863-893.
17.Lau, C.H., Springston, E.E. , Sohn, M.W., Mason, I., Gadola, E., Damitza, M., and Gupta, R.S. 2012. Hand hygiene instruction decreases illness-related absenteeism in elementary schools: a prospective cohort study. Biomedical Central Pediatrics, 12 (52): 1-7. 18.Leaper, D., McBainm, A.J., Kramer, A., Assdian, O., Sanchez, J.L.A., Lumio, J, and Kiernan, M. 2010. Healthcare associated infection: novel strategies and antimicrobial implants to prevent surgical site infection. Anals of the Royal College of Surgeons of England, 92: 452-458.
19.Mangalappalli-Illathu, A.K. , and Korber, D.R. 2006. Adaptive resistance and differential protein expression on Salmonella enteric serovar enteritidis biofilms exposed to benzalkonium chloride. Antimicrobial agents and chemotherapy, 50 (11): 3588-3596.
20.McDonnell, G., and Russell, A.D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews, 12 (1): 147-179.
21.Moen, B., Rudi, K., Bore, E., and Langsrud, S. 2012. Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of E. coli with inheritable characteristics. International Journal of Molecular Sciences, 12: 4101-4123.
22.Nasvall, J., Sun, L., Roth, J.R., and Andersson, D.I. 2012. Real-time evolution of new genes by innovation, amplification and divergence. Science, 338 (6105):384-387.
23.Nic, M., Jirat, B., and Kosata, B. 2006. IUPAC of quaternary ammonium compounds [online]. Available from http://goldbook.iupac.org/Q05003.html [accessed 26 October 2012].
24.Oxford, J.S., Potter, W., McLaren, C., and Hardy, W. 1971. Inactivation of influenza and other viruses by a mixture of virucidal compounds. American Society for Microbiology, 21 (4): 606-610.
25.Pagear, A., Singh, J., and Batish, V.K. 2011. Efflux mediated adaptive and cross resistance to ciprofloxacin and benzalkonium chloride in Pseudomonas aeruginosa of dairy origin. Journal of Basic Microbiology, 51: 289-295.
26.Prazuck, T., Compte-Nguyen, G., Pelat, C., Sunder, S., and Blanchon, T. 2010. Reducing gastroenteritis occurrences and their consequences in elementary schools with alcohol-based hand sanitizers. The Pediatric Infectious Disease Journal, 29 (11): 994-998. 27.Reynolds, S.A., Levy, F., and Walker, E.S. 2006. Hand sanitizer alert. Emerging Infectious Diseases, 12 (3): 527-529.
28.Sidhu, M.S., Heir, E., Sorum, and Holck A. 2001. Genetic linkage between resistance to quaternary ammonium compounds and beta-lactam antibiotics in food-related Staphylococcus spp. Microbial Drug Resistance, 7 (4): 363-371.
29.Sheldon, A.T. 2005. Antiseptic “resistance”: real or perceived threat? Clinical Infectious Diseases, 40 (11): 1650-1656.
30.To, M.S., Favrin, S., Romanova, N., and Griffiths, M.W. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Applied and Environmental Microbiology, 68 (11): 5258-5264.
31.White, C., Kolble, R., Carlson, R., Lipson, N., Dolan, M., Ali, Y., and Cline, M. 2003. The effect of hand hygiene on illness rate among students in university residence halls. American Journal of Infection Control, 31:364–370.
32.MICB 201 textbook citation??????