West Nile Virus and Acute Flaccid Paralysis: Difference between revisions
| Line 9: | Line 9: | ||
<br>Current research focuses on preventing future epidemics as well as the development of a vaccine for West Nile Virus. In the meantime, efforts to survey outbreaks as well as limit human exposure are utilized to prevent possible outbreaks of the disease. Emerging research also continues to explore the fatal neuroinvasive diseases associated with West Nile Virus in the attempts to prevent and eradicate these as well. | <br>Current research focuses on preventing future epidemics as well as the development of a vaccine for West Nile Virus. In the meantime, efforts to survey outbreaks as well as limit human exposure are utilized to prevent possible outbreaks of the disease. Emerging research also continues to explore the fatal neuroinvasive diseases associated with West Nile Virus in the attempts to prevent and eradicate these as well. | ||
==Structure and Genome== | |||
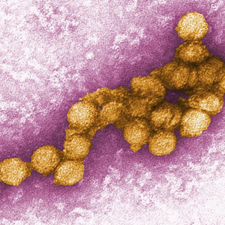
<br>West Nile virus is a spherical virus that possesses a membrane-enclosed nucelocapsid core and is typically between 40 and 50nm in diameter. The West Nile virions are icosahedral, meaning the genome is contained in a 20-face polyhedron capsid (Iyer et al. 2013). The genome is positive single stranded RNA that is approximately 11kb in size. As a member of Group IV of the Baltimore classification system of viruses, West Nile virus is made up of a positive sense-coding strand that can be directly translated into one viral polyprotein. This virus does not have the ability to replicate RNA directly however, and must rely on other RNA polymerases to complete this task. West Nile virus contains codes for three structural proteins, including proteins that make up the envelope, membrane, and capsid, as well as seven proteins that are unrelated to the structure (Sejvar et al. 2005). The envelope protein (E) is particularly interesting and important for viral transmission because it acts as a surface protein that allows for attachment onto host cells, subsequent membrane penetration, fusion, and assembly of West Nile virions. Without this protein the virus would not be able to insert into host cells and complete replication (Sejvar et al.2005). | |||
==Viral Life Cycle== | |||
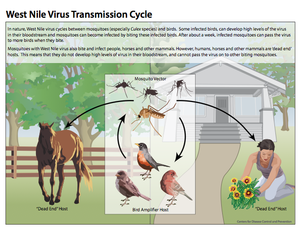
[[Image:WNVtransmissioncycle.png|thumb|300px|right|This diagram illustrates the West Nile virus life cycle, including transmission patterns. This image is made available by the Centers for Disease Control and Prevention]] | [[Image:WNVtransmissioncycle.png|thumb|300px|right|This diagram illustrates the West Nile virus life cycle, including transmission patterns. This image is made available by the Centers for Disease Control and Prevention]] | ||
<br>West Nile virus typically cycles between birds and mosquitoes, with the birds acting as the amplifier host and the mosquitoes acting as the vectors (Figure 2) (Girard et al. 2005). Studies have shown that the virus is maintained in mosquitoes during the winter months, after which, the enzootic cycle begins. This occurs when mosquitoes carrying West Nile virus feed on birds and transmit the disease to the avian hosts. Infected birds generate high levels of the disease, known as high titer viremia, which allows for the virus to be passed to uninfected mosquitoes once they feed on these birds (Girard et al. 2005). Infected mosquitoes continue to pass the virus on to every subsequent organism they come into contact with and feed on. Ultimately, once the prevalence of infected avian hosts and mosquitoes in a certain area reaches a high enough volume, mosquitoes that primarily feed on mammals and other organisms besides birds become infected as well. It is via these mosquito species that humans and other large mammals become infected (Nasci et al. 2013). However, unlike infected birds, humans and other organisms such as horses are dead-end hosts, meaning uninfected mosquitoes cannot acquire the virus from these hosts and the cycle ceases from this point (Nasci et al. 2013). | |||
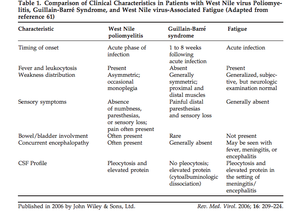
[[Image:Table1GBAFC.png|thumb|300px|right|This table compares characteristics of West Nile poliomyelitis with Guillian-Barre syndrome. West Nile poliomyelitis is often confused for and misdiagnosed as Guillian-Barre Syndrome. This table represents the work completed by Sejvar et al. 2006 published in Reviews in Medical Virology.]] | [[Image:Table1GBAFC.png|thumb|300px|right|This table compares characteristics of West Nile poliomyelitis with Guillian-Barre syndrome. West Nile poliomyelitis is often confused for and misdiagnosed as Guillian-Barre Syndrome. This table represents the work completed by Sejvar et al. 2006 published in Reviews in Medical Virology.]] | ||
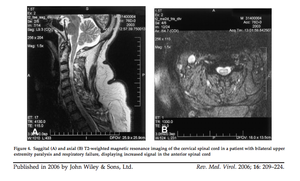
[[Image:ACFspinalcord.png|thumb|300px|right|Saggital (A) and axial (B) T2-weighted magnetic resonance imaging of the cervical spinal cord in a patient with bilateral upper extremity paralysis and respiratory failure, displaying increased signal in the anterior spinal cord. Sejvar et al. 2006.]] | [[Image:ACFspinalcord.png|thumb|300px|right|Saggital (A) and axial (B) T2-weighted magnetic resonance imaging of the cervical spinal cord in a patient with bilateral upper extremity paralysis and respiratory failure, displaying increased signal in the anterior spinal cord. Sejvar et al. 2006.]] | ||
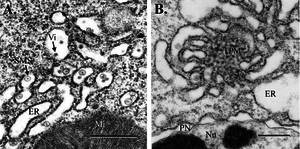
[[Image:midgutepithelium.png|thumb|300px|right|Fig. 1. Mosquito midgut epithelium, 3 d postinfection (bar, 0.5 μm). (A) WNV virions and SMS in cisternae of the epithelial RER. (B) TPM structure and dilated RER associated with WNV replication. Mi, mitochondrion; Nu, nucleus; PN, perinuclear space; Vi, virion]] | [[Image:midgutepithelium.png|thumb|300px|right|Fig. 1. Mosquito midgut epithelium, 3 d postinfection (bar, 0.5 μm). (A) WNV virions and SMS in cisternae of the epithelial RER. (B) TPM structure and dilated RER associated with WNV replication. Mi, mitochondrion; Nu, nucleus; PN, perinuclear space; Vi, virion]] | ||
Revision as of 01:30, 23 April 2014
Background
By Kate Lang
Viruses as a whole are widespread and diverse, especially because they are transmitted via many vectors and cause a variety of symptoms. Many dangerous viruses are transmitted via insects (arthropods) such as mosquitoes. The most common arbovirus, meaning arthropod-borne virus, worldwide is West Nile Virus (WNV), and recent epidemics have brought this virus into the global spotlight as an emerging threat requiring immediate attention for future prevention (Campel et al. 2002).
West Nile virus (Flavividae) is a member of the family Flavivirus and is in the same serocomplex (antigen group) as Japanese encephalitis virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus (Sejvar et al. 2006). Other well-known flaviviruses from this family include Yellow Fever and Dengue Fever, which are also mosquito-borne pathogens (CDC, Flavivirus). West Nile Virus was first identified in the United States in New York in 1999, and since this introduction, it has spread all across the lower 48 states, becoming endemic. Cases of West Nile Virus are particularly concentrated in the Midwestern and Rocky Mountain states during the mid to late summer; however incidences have appeared all across the country year round (Nasci et al. 2103).
Patients infected with West Nile develop a wide range of symptoms, though in most cases no symptoms arise at all. Out of those infected with West Nile virus, 70-80% are asymptomatic and approximately one quarter develops West Nile fever, which results in a wide spectrum of relatively minor symptoms (Sejvar et al. 2006). However, in rare cases (less than 1%) certain people develop severe neurologic disease, which includes encephalitis, meningitis, and acute flaccid paralysis (Burton et al. 2004). These neurologic disorders tend to be very dangerous and carry a relatively high fatality rate of 10% (Sejvar et al. 2005).
Current research focuses on preventing future epidemics as well as the development of a vaccine for West Nile Virus. In the meantime, efforts to survey outbreaks as well as limit human exposure are utilized to prevent possible outbreaks of the disease. Emerging research also continues to explore the fatal neuroinvasive diseases associated with West Nile Virus in the attempts to prevent and eradicate these as well.
Structure and Genome
West Nile virus is a spherical virus that possesses a membrane-enclosed nucelocapsid core and is typically between 40 and 50nm in diameter. The West Nile virions are icosahedral, meaning the genome is contained in a 20-face polyhedron capsid (Iyer et al. 2013). The genome is positive single stranded RNA that is approximately 11kb in size. As a member of Group IV of the Baltimore classification system of viruses, West Nile virus is made up of a positive sense-coding strand that can be directly translated into one viral polyprotein. This virus does not have the ability to replicate RNA directly however, and must rely on other RNA polymerases to complete this task. West Nile virus contains codes for three structural proteins, including proteins that make up the envelope, membrane, and capsid, as well as seven proteins that are unrelated to the structure (Sejvar et al. 2005). The envelope protein (E) is particularly interesting and important for viral transmission because it acts as a surface protein that allows for attachment onto host cells, subsequent membrane penetration, fusion, and assembly of West Nile virions. Without this protein the virus would not be able to insert into host cells and complete replication (Sejvar et al.2005).
Viral Life Cycle
West Nile virus typically cycles between birds and mosquitoes, with the birds acting as the amplifier host and the mosquitoes acting as the vectors (Figure 2) (Girard et al. 2005). Studies have shown that the virus is maintained in mosquitoes during the winter months, after which, the enzootic cycle begins. This occurs when mosquitoes carrying West Nile virus feed on birds and transmit the disease to the avian hosts. Infected birds generate high levels of the disease, known as high titer viremia, which allows for the virus to be passed to uninfected mosquitoes once they feed on these birds (Girard et al. 2005). Infected mosquitoes continue to pass the virus on to every subsequent organism they come into contact with and feed on. Ultimately, once the prevalence of infected avian hosts and mosquitoes in a certain area reaches a high enough volume, mosquitoes that primarily feed on mammals and other organisms besides birds become infected as well. It is via these mosquito species that humans and other large mammals become infected (Nasci et al. 2013). However, unlike infected birds, humans and other organisms such as horses are dead-end hosts, meaning uninfected mosquitoes cannot acquire the virus from these hosts and the cycle ceases from this point (Nasci et al. 2013).