West Nile Virus and Acute Flaccid Paralysis: Difference between revisions
No edit summary |
|||
| (27 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Background== | ==Background== | ||
<br>By Kate Lang<br> | <br>By Kate Lang<br> | ||
[[Image:flaviviridae1.jpeg|thumb|300px|right|Figure 1:Electron micrograph image of West Nile virus. This image is made public courteous of the Centers for Disease Control and Prevention, specifically the Viral Special Pathogens Branch. [http://www.news-medical.net/image.axd?picture=transmission%20electron%20micrograph%20(TEM)%20of%20the%20West%20Nile%20virus.jpg ( | [[Image:flaviviridae1.jpeg|thumb|300px|right|Figure 1:Electron micrograph image of West Nile virus. This image is made public courteous of the Centers for Disease Control and Prevention, specifically the Viral Special Pathogens Branch. [http://www.news-medical.net/image.axd?picture=transmission%20electron%20micrograph%20(TEM)%20of%20the%20West%20Nile%20virus.jpg (CDC, West Nile virus.)].]] | ||
<br>Viruses are widespread and diverse, are transmitted via many vectors, and cause a variety of illnesses. Many dangerous viruses are transmitted via insects (arthropods) such as mosquitoes. The most common arbovirus (arthropod-borne virus) worldwide is West Nile | <br>Viruses are widespread and diverse, are transmitted via many vectors, and cause a variety of illnesses. Many dangerous viruses are transmitted via insects (arthropods) such as mosquitoes. The most common arbovirus (arthropod-borne virus) worldwide is West Nile virus (WNV). Recent epidemics have brought this virus into the global spotlight as an emerging threat requiring immediate attention for future prevention (Campbell <i>et al.</i> 2002). | ||
<br>West Nile virus (Flavividae) is a member of the family Flavivirus and is in the same serocomplex (antigen group) as Japanese encephalitis virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus (Sejvar <i>et al.</i> 2006). Other well-known flaviviruses from this family include Yellow Fever and Dengue Fever, which are also mosquito-borne pathogens (CDC, Flavivirus). West Nile | <br>West Nile virus (Flavividae) is a member of the family Flavivirus and is in the same serocomplex (antigen group) as Japanese encephalitis virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus (Sejvar <i>et al.</i> 2006). Other well-known flaviviruses from this family include Yellow Fever and Dengue Fever, which are also mosquito-borne pathogens (CDC, Flavivirus). West Nile virus was first introduced in the United States in New York in 1999, and has since spread all across the lower 48 states, becoming endemic. Cases of West Nile virus are particularly concentrated in the Midwest and Rocky Mountain states during the mid to late summer; however infections have appeared all across the country (Nasci <i>et al.</i> 2013). | ||
<br>Patients infected with West Nile develop a wide range of symptoms, though in most cases no symptoms or only very mild symptoms occur. Of those infected with West Nile virus, 70-80% are asymptomatic and approximately 25% develop West Nile fever, which results in a wide spectrum of relatively minor symptoms (Sejvar <i>et al.</i> 2006). However, in rare cases (less than 1%) certain people develop severe neurologic disease, which includes encephalitis, meningitis, and acute flaccid paralysis (Burton <i>et al.</i> 2004). These neurologic disorders tend to be very severe and carry a relatively high fatality rate of 10% (Sejvar <i>et al.</i> 2005). | <br>Patients infected with West Nile develop a wide range of symptoms, though in most cases no symptoms or only very mild symptoms occur. Of those infected with West Nile virus, 70-80% are asymptomatic and approximately 25% develop West Nile fever, which results in a wide spectrum of relatively minor symptoms (Sejvar <i>et al.</i> 2006). However, in rare cases (less than 1%) certain people develop severe neurologic disease, which includes encephalitis, meningitis, and acute flaccid paralysis (Burton <i>et al.</i> 2004). These neurologic disorders tend to be very severe and carry a relatively high fatality rate of 10% (Sejvar <i>et al.</i> 2005). | ||
<br>Current research involving West Nile | <br>Current research involving West Nile virus focuses on preventing future epidemics as well as the development of a vaccine. In the meantime, efforts to survey outbreaks as well as limit human exposure are utilized to prevent possible outbreaks of the disease. Emerging research also continues to explore the often fatal neuroinvasive infections due to West Nile virus in the attempts to prevent and treat these as well. | ||
==Structure and Genome== | ==Structure and Genome== | ||
| Line 14: | Line 15: | ||
==Viral Life Cycle== | ==Viral Life Cycle== | ||
[[Image:WNVtransmissioncycle.png|thumb|300px|right|Figure 2:This diagram illustrates the West Nile virus life cycle, including transmission patterns. This image is made available by the Centers for Disease Control and Prevention. [http://www.cdc.gov/westnile/resources/pdfs/13_240124_west_nile_lifecycle_birds_plainlanguage_508.pdf ( | [[Image:WNVtransmissioncycle.png|thumb|300px|right|Figure 2:This diagram illustrates the West Nile virus life cycle, including transmission patterns. This image is made available by the Centers for Disease Control and Prevention. [http://www.cdc.gov/westnile/resources/pdfs/13_240124_west_nile_lifecycle_birds_plainlanguage_508.pdf (CDC, West Nile virus)]]] | ||
<br>West Nile virus typically cycles between birds and mosquitoes, with the birds acting as the amplifier host and the mosquitoes acting as the vectors (Figure 2) (Girard <i>et al.</i> 2005). Studies have shown that the virus is maintained in mosquitoes during the winter months, after which, the enzootic cycle begins. This occurs when mosquitoes carrying West Nile virus feed on birds and transmit the disease to the avian hosts. Infected birds generate high levels of infection, known as high titer viremia, which allows for the virus to be passed to uninfected mosquitoes once they feed on these birds (Girard <i>et al.</i> 2005). Infected mosquitoes continue to pass the virus on to every organism they come into contact with and feed on. Ultimately, once the prevalence of infected avian hosts and mosquitoes in a certain area reaches a high enough level, mosquitoes that primarily feed on mammals and other organisms besides birds become infected as well. It is via these mosquito species that humans and other large mammals become infected (Nasci <i>et al.</i> 2013). However, unlike infected birds, humans and other organisms such as horses are dead-end hosts. These hosts do not generate high enough viral loads, therefore uninfected mosquitoes cannot acquire the virus from these hosts and the cycle hits a "dead end" (Nasci <i>et al.</i> 2013). | <br>West Nile virus typically cycles between birds and mosquitoes, with the birds acting as the amplifier host and the mosquitoes acting as the vectors (Figure 2) (Girard <i>et al.</i> 2005). Studies have shown that the virus is maintained in mosquitoes during the winter months, after which, the enzootic cycle begins. This occurs when mosquitoes carrying West Nile virus feed on birds and transmit the disease to the avian hosts. Infected birds generate high levels of infection, known as high titer viremia, which allows for the virus to be passed to uninfected mosquitoes once they feed on these birds (Girard <i>et al.</i> 2005). Infected mosquitoes continue to pass the virus on to every susceptible organism they come into contact with and feed on. Ultimately, once the prevalence of infected avian hosts and mosquitoes in a certain area reaches a high enough level, mosquitoes that primarily feed on mammals and other organisms besides birds become infected as well. It is via these mosquito species that humans and other large mammals become infected (Nasci <i>et al.</i> 2013). However, unlike infected birds, humans and other organisms such as horses are dead-end hosts. These hosts do not generate high enough viral loads, therefore uninfected mosquitoes cannot acquire the virus from these hosts and the cycle hits a "dead end" (Nasci <i>et al.</i> 2013). | ||
==Transmission to Human Hosts== | ==Transmission to Human Hosts== | ||
[[Image:mosquitownv.jpg|thumb|300px|right|Figure 3: Image of mosquito containing West Nile virus infected host blood.[http://assets.nydailynews.com/polopoly_fs/1.1122249!/img/httpImage/image.jpg_gen/derivatives/article_970/nile27n-1-web.jpg] ]] | [[Image:mosquitownv.jpg|thumb|300px|right|Figure 3: Image of mosquito containing West Nile virus infected host blood.[http://assets.nydailynews.com/polopoly_fs/1.1122249!/img/httpImage/image.jpg_gen/derivatives/article_970/nile27n-1-web.jpg (USDA)]]] | ||
<br><b>Vectors</b> | <br><b>Vectors</b> | ||
<br>Mosquitoes, particularly members of the <i>Culex</i> family, act as the primary vectors of West Nile virus. The most common mosquito vectors are <i>Culex pipiens pipiens, Culex pipiens quinquefasciatus</i>, and <i>Culex tarsalis</i> (Figure 3)(CDC, West Nile Virus). However, at least 58 species of mosquitoes have been identified as major vectors for West Nile virus in North America (Sevjay et al. 2006). The staggering number of mosquito populations covering the contiguous United States allows for easy transmission of West Nile virus in numerous locations across the country. | <br>Mosquitoes, particularly members of the <i>Culex</i> family, act as the primary vectors of West Nile virus. The most common mosquito vectors are <i>Culex pipiens pipiens, Culex pipiens quinquefasciatus</i>, and <i>Culex tarsalis</i> (Figure 3)(CDC, West Nile Virus). However, at least 58 species of mosquitoes have been identified as major vectors for West Nile virus in North America (Sevjay et al. 2006). The staggering number of mosquito populations covering the contiguous United States allows for easy transmission of West Nile virus in numerous locations across the country. | ||
[[Image:midgutepithelium.png|thumb|300px|right|Figure 4. Mosquito midgut epithelium, 3 d postinfection (bar, 0.5 μm). (A) WNV virions and SMS in cisternae of the epithelial RER. (B) TPM structure and dilated RER associated with WNV replication. Mi, mitochondrion; Nu, nucleus; PN, perinuclear space; Vi, virion. [http://www.ncbi.nlm.nih.gov/pubmed/15962797 ( | [[Image:midgutepithelium.png|thumb|300px|right|Figure 4. Mosquito midgut epithelium, 3 d postinfection (bar, 0.5 μm). (A) WNV virions and SMS in cisternae of the epithelial RER. (B) TPM structure and dilated RER associated with WNV replication. Mi, mitochondrion; Nu, nucleus; PN, perinuclear space; Vi, virion. [http://www.ncbi.nlm.nih.gov/pubmed/15962797 (Girard <i>et al.</i>2005)]]] | ||
<br>The vector mosquitoes become infected with the virus after feeding on host animals, such as birds, that are carrying the virus. Three days after the infected blood is ingested by the mosquito, the virus | <br>The vector mosquitoes become infected with the virus after feeding on host animals, such as birds, that are carrying the virus. Three days after the infected blood is ingested by the mosquito, the virus begins to replicate in the mosquitoes’ midgut epithelial cells-particularly in the rough endoplasmic reticulum cisternae. The virus then escapes through the basal lamina, particularly in the fatty regions, and is not found in the muscle tissue until much later (Girard <i>et al.</i> 2005). Infection of the midgut results in several morphological changes in the midgut, such as membrane proliferation and swelling of the cisternae (Figure 4). The most notable change, however, is the concentration of smooth membrane structures in the lumen of the rough endoplasmic reticulum. By 21 days post infection, high levels of West Nile virus replication can be detected in the midgut muscle, and vacuoles containing high levels of virions are expelled (Girard <i>et al.</i> 2005). Viral replication occurs in other areas of the mosquito as well, particularly the brain and salivary glands. However, replication consistently occurs primarily in the fatty cells no matter the location in the mosquito. After the virus has replicated inside the mosquito it can be passed to birds or to dead end hosts such as humans once the mosquito feeds on these new organisms (Nasci <i>et al.</i> 2013). | ||
<br><b>Non-Vector Transmission</b> | <br><b>Non-Vector Transmission</b> | ||
| Line 37: | Line 38: | ||
==Pathology== | ==Pathology== | ||
[[Image:dynamicsofWNV.png|thumb|300px|right|Figure 5:Dynamics of West Nile virus (WNV) RNA and WNV-specific antibody positivity and negativity. The top 3 intervals represent window periods that start with the index donation (i.e. the donation that tested positive for WNV in minipools of 16 donor specimens [MP] by transcription-mediated amplification [MP-TMA]). Although positive results of MP-TMA can occur anytime during the 6.9-day window period when results of MP nucleic acid amplification testing (NAT) are positive, for illustrative purposes the first 3 window periods are depticted as beginning at the midpoint of this window period. Values are not additive (i.e. the time from RNA detection to IgM detection [3.9 days] plus the time from IgM antibody to IgG antibody detection [3.4 days] does not equal the time from RNA detection to IgG antibody detection [7.7 days]) as noted in table 2. ID, individual donation; 1 x ID, single replicate; 6 x ID, 6 replicates.[http://web.b.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=c0072353-e92c-4841-9af8-31c5473db949%40sessionmgr110&vid=2&hid=120 ( | [[Image:dynamicsofWNV.png|thumb|300px|right|Figure 5:Dynamics of West Nile virus (WNV) RNA and WNV-specific antibody positivity and negativity. The top 3 intervals represent window periods that start with the index donation (i.e. the donation that tested positive for WNV in minipools of 16 donor specimens [MP] by transcription-mediated amplification [MP-TMA]). Although positive results of MP-TMA can occur anytime during the 6.9-day window period when results of MP nucleic acid amplification testing (NAT) are positive, for illustrative purposes the first 3 window periods are depticted as beginning at the midpoint of this window period. Values are not additive (i.e. the time from RNA detection to IgM detection [3.9 days] plus the time from IgM antibody to IgG antibody detection [3.4 days] does not equal the time from RNA detection to IgG antibody detection [7.7 days]) as noted in table 2. ID, individual donation; 1 x ID, single replicate; 6 x ID, 6 replicates.[http://web.b.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=c0072353-e92c-4841-9af8-31c5473db949%40sessionmgr110&vid=2&hid=120 (Busch <i>et al.</i> 2008)]]] | ||
<br>The dynamics of West Nile virus infection in the human body have been the topic of recent studies. The work completed by Busch <i>et al.</i> 2008 explored the early stages of infection in order to improve early detection as well as prevention techniques. In doing so, these researchers also gained an in depth understanding of the activity of the virus in the body and the body's response. In this experiment, 245 donors were sampled over the course of a six month period for West Nile virus viral load and antibody production. Using these data, Busch and colleagues were able to generate an accurate model of viral dynamics, including the amount of time it took the body to generate an immune response (as indicated by the production of West Nile virus-specific IgM and IgG antibodies (Figure 5). They were able to detect lower levels of viremia after less than a day post-exposure, as well as show that antibodies are typically generated in the body after approximately 4-7 days- IgM seroconversion occurs before IgG. Further work analyzing the body's response to infection by West Nile virus is important as much is | <br>The dynamics of West Nile virus infection in the human body have been the topic of recent studies. The work completed by Busch <i>et al.</i> 2008 explored the early stages of infection in order to improve early detection as well as prevention techniques. In doing so, these researchers also gained an in depth understanding of the activity of the virus in the body and the body's response. In this experiment, 245 donors were sampled over the course of a six month period for West Nile virus viral load and antibody production. Using these data, Busch and colleagues were able to generate an accurate model of viral dynamics, including the amount of time it took the body to generate an immune response (as indicated by the production of West Nile virus-specific IgM and IgG antibodies (Figure 5). They were able to detect lower levels of viremia after less than a day post-exposure, as well as show that antibodies are typically generated in the body after approximately 4-7 days- IgM seroconversion occurs before IgG. Further work analyzing the body's response to infection by West Nile virus is important, as there is still much that is unknown. | ||
<br>After infection with West Nile virus, it takes approximately two to four days before viremia levels peak in human hosts. Currently the invasion mechanism utilized by the virus for entry into the Central Nervous System is unknown, however researchers speculate the virus is able to invade cerebral endothelial cells before migrating throughout the brain. After the virus has propagated throughout the body, symptoms will begin to manifest in 25% of cases (Sejvar, 2014). | <br>After infection with West Nile virus, it takes approximately two to four days before viremia levels peak in human hosts. Currently the invasion mechanism utilized by the virus for entry into the Central Nervous System is unknown, however researchers speculate the virus is able to invade cerebral endothelial cells before migrating throughout the brain. After the virus has propagated throughout the body, symptoms will begin to manifest in 25% of cases (Sejvar, 2014). | ||
| Line 45: | Line 46: | ||
<br>The majority of people who are infected with West Nile virus are asymptomatic (approximately 70-80% of cases). In these cases the infected persons are likely completely unaware they were even exposed to the virus (Sejvar, 2014). | <br>The majority of people who are infected with West Nile virus are asymptomatic (approximately 70-80% of cases). In these cases the infected persons are likely completely unaware they were even exposed to the virus (Sejvar, 2014). | ||
<br>In approximately 20-25% of cases, patients develop febrile illness and a variety of accompanying symptoms. This is known as West Nile fever and it has a typical incubation period of 2-14 days before the development of symptoms. The symptoms of West Nile fever are body aches (myalgias) and joint pains, as well as headaches, vomiting, diarrhea and a rash. The appearance of a rash has been found more frequently in patients | <br>In approximately 20-25% of cases, patients develop febrile illness and a variety of accompanying symptoms. This is known as West Nile fever and it has a typical incubation period of 2-14 days before the development of symptoms. The symptoms of West Nile fever are body aches (myalgias) and joint pains, as well as headaches, vomiting, diarrhea and a rash. The appearance of a rash has been found more frequently in younger patients, which leads medical researchers to question whether host or immune response dictates the probability of a rash developing (Sejvar, 2014). Patients also experience a relatively rapid onset of fever. Overall, these symptoms can range from mild to more severe, however they typically disappear after a few weeks. In some cases, patients will suffer from prolonged fatigue for months after any other symptoms have disappeared. Treatment for the West Nile febrile illness is limited to over-the-counter pain relievers and any other mild analgesics that temporarily relieve basic symptoms and pain. Though there is technically no cure, most patients make a complete recovery after a few months. Death resulting from West Nile fever mainly threatens elderly patients or those who are already immunocompromised (Sejvar, 2014). | ||
<br>In rare cases, less than 1%, patients will develop severe symptoms as a result of dangerous neurological illnesses. These neuroinvasive diseases include encephalitis (inflammation of the brain), aseptic meningitis (inflammation of the spinal cord and surrounding tissue), and acute flaccid paralysis (Frih-Ayed <i>et al.</i> 2005). Patients suffering from neurologic infection can exhibit a variety of symptoms, including headaches, seizures, and altered mental status, such as coma. This can be particularly dangerous for the elderly as well as those living with other medical problems, such as cancers and cardiopulmonary diseases. Patients must be treated on a case-by-case basis to alleviate symptoms, and hospitalization is commonly required for those suffering from the most severe symptoms and neurological infections. These potential neuroinvasive diseases that result from West Nile virus pose a serious risk, and the observed prevalence of these diseases has continued to mount in the past decade (Sejvar, 2014). Acute flaccid paralysis, which is emerging as a focal point of research, is explored in depth below. | <br>In rare cases, less than 1%, patients will develop severe symptoms as a result of dangerous neurological illnesses. These neuroinvasive diseases include encephalitis (inflammation of the brain), aseptic meningitis (inflammation of the spinal cord and surrounding tissue), and acute flaccid paralysis (Frih-Ayed <i>et al.</i> 2005). Patients suffering from neurologic infection can exhibit a variety of symptoms, including headaches, seizures, and altered mental status, such as coma. This can be particularly dangerous for the elderly as well as those living with other medical problems, such as cancers and cardiopulmonary diseases. Patients must be treated on a case-by-case basis to alleviate symptoms, and hospitalization is commonly required for those suffering from the most severe symptoms and neurological infections. These potential neuroinvasive diseases that result from West Nile virus pose a serious risk, and the observed prevalence of these diseases has continued to mount in the past decade (Sejvar, 2014). Acute flaccid paralysis, which is emerging as a focal point of research, is explored in depth below. | ||
==Acute Flaccid Paralysis== | ==Acute Flaccid Paralysis== | ||
[[Image:ACFspinalcord.png|thumb|300px|right|Figure 6: Saggital (A) and axial (B) T2-weighted magnetic resonance imaging of the cervical spinal cord in a patient with bilateral upper extremity paralysis and respiratory failure, displaying increased signal in the anterior spinal cord. [http://onlinelibrary.wiley.com/store/10.1002/rmv.501/asset/501_ftp.pdf?v=1&t=hux9cr29&s=aeac98d4b68b656bbdecb047661676a7a7ee0feb ( | [[Image:ACFspinalcord.png|thumb|300px|right|Figure 6: Saggital (A) and axial (B) T2-weighted magnetic resonance imaging of the cervical spinal cord in a patient with bilateral upper extremity paralysis and respiratory failure, displaying increased signal in the anterior spinal cord. [http://onlinelibrary.wiley.com/store/10.1002/rmv.501/asset/501_ftp.pdf?v=1&t=hux9cr29&s=aeac98d4b68b656bbdecb047661676a7a7ee0feb (Sejvar <i>et al.</i> 2005)]]] | ||
<br>Among the neuroinvasive diseases associated with West Nile virus, acute flaccid paralysis is one of the least well studied, however it is emerging as a serious problem for those afflicted with the virus. Acute flaccid paralysis, or “West Nile poliomyelitis,” refers to the relatively sudden and asymmetrical loss of control and function of localized areas of the body due to infection by the West Nile virus. Recent research suggests that this is the result of viral infection of anterior horn cells, which are motor neurons located in the lower spinal cord (Sejvar et al. 2005). Lab-induced infectious interference of these cells has been shown to cause | <br>Among the neuroinvasive diseases associated with West Nile virus, acute flaccid paralysis is one of the least well studied, however it is emerging as a serious problem for those afflicted with the virus. Acute flaccid paralysis, or “West Nile poliomyelitis,” refers to the relatively sudden and asymmetrical loss of control and function of localized areas of the body due to infection by the West Nile virus. Recent research suggests that this is the result of viral infection of anterior horn cells, which are motor neurons located in the lower spinal cord (Sejvar <i>et al.</i> 2005). Lab-induced infectious interference of these cells has been shown to cause symptoms identical to the paralysis seen resulting in acute flaccid paralysis (Figure 6). Due to the fact that this manifestation is so rare, it is often confused with Guillan-Barré syndrome and poliovirus. Additionally, a prominent symptom of West Nile fever is fatigue, however the severity of the weakness from acute flaccid paralysis clearly differentiate it from the fatigue commonly associated with West Nile fever (Figure 7)(Sejvar, 2014). Several recent studies have examined specific cases of acute flaccid paralysis in order to gain a better understanding of what causes the symptoms, as well as how to control and prevent this paralysis in the future. | ||
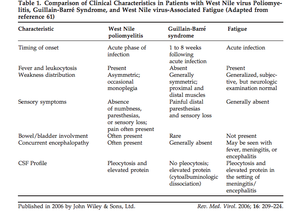
[[Image:Table1GBAFC.png|thumb|300px|right|Figure 7:This table compares characteristics of West Nile poliomyelitis with Guillian-Barre syndrome. West Nile poliomyelitis is often confused for and misdiagnosed as Guillian-Barre Syndrome. This table represents the work completed by Sejvar et al. 2006 published in Reviews in Medical Virology.[http://onlinelibrary.wiley.com/store/10.1002/rmv.501/asset/501_ftp.pdf?v=1&t=hux9cr29&s=aeac98d4b68b656bbdecb047661676a7a7ee0feb ( | [[Image:Table1GBAFC.png|thumb|300px|right|Figure 7:This table compares characteristics of West Nile poliomyelitis with Guillian-Barre syndrome. West Nile poliomyelitis is often confused for and misdiagnosed as Guillian-Barre Syndrome. This table represents the work completed by Sejvar et al. 2006 published in Reviews in Medical Virology.[http://onlinelibrary.wiley.com/store/10.1002/rmv.501/asset/501_ftp.pdf?v=1&t=hux9cr29&s=aeac98d4b68b656bbdecb047661676a7a7ee0feb (Sejvar <i>et al.</i> 2005)]]] | ||
<br>Onset of paralysis symptoms occurs quickly post-infection- typically between 24 and 48 hours (Sevjar <i>et al.</i> 2006). The paralysis tends to be asymmetrical, and most patients lose function or experience weakness in one limb, though some will experience it in multiple limbs. Changes in facial sensation as well as bilateral facial weakness are common. Perhaps the most dangerous symptom that accompanies acute flaccid paralysis is the disruption of respiratory function as a result of the paralysis of diaphragmatic and intercostal muscles (Sevjar, 2014). | <br>Onset of paralysis symptoms occurs quickly post-infection- typically between 24 and 48 hours (Sevjar <i>et al.</i> 2006). The paralysis tends to be asymmetrical, and most patients lose function or experience weakness in one limb, though some will experience it in multiple limbs. Changes in facial sensation as well as bilateral facial weakness are common. Perhaps the most dangerous symptom that accompanies acute flaccid paralysis is the disruption of respiratory function as a result of the paralysis of diaphragmatic and intercostal muscles (Sevjar, 2014). | ||
<br>The work completed by Saad <i>et al.</i> examined 3 individual case studies of acute flaccid paralysis and West Nile virus as well as accumulated data on 56 other cases of West Nile virus-induced acute flaccid paralysis. They found that 86% of cases of acute flaccid paralysis due to West Nile virus occurred in people over the age of 40, and 35 out of the 56 people presented with a wide variety of other underlying diseases or health problems, including COPD, previous organ transplants, and cancer. A large majority of patients reported fevers (92%) whereas only 17% presented with nuchal rigidity (menigismus). A surprising number of patients experienced weakness in all four limbs (56%), and overall there was a mortality rate of 22%, predominately as a result of respiratory failure. | <br>The work completed by Saad <i>et al.</i> examined 3 individual case studies of acute flaccid paralysis and West Nile virus as well as accumulated data on 56 other cases of West Nile virus-induced acute flaccid paralysis. They found that 86% of cases of acute flaccid paralysis due to West Nile virus occurred in people over the age of 40, and 35 out of the 56 people presented with a wide variety of other underlying diseases or health problems, including COPD, previous organ transplants, and cancer. A large majority of patients reported fevers (92%) whereas only 17% presented with nuchal rigidity (menigismus). A surprising number of patients experienced weakness in all four limbs (56%), and overall there was a mortality rate of 22%, predominately as a result of respiratory failure. | ||
<br>Overall, current data suggest that patients who acquire acute flaccid paralysis as a result of West Nile virus | <br>Overall, current data suggest that patients who acquire acute flaccid paralysis as a result of West Nile virus undergo a difficult and extensive recovery period that is rarely completely successful. Motor control and strength recovery in effected limbs varies between patients, and physical therapy is typically required for a long period of time after infection. The mortality rate of acute flaccid paralysis is relatively high, particularly for elderly and immunocompromised individuals. The main cause of mortality is respiratory failure (50% of deaths are due to acute neuromuscular respiratory failure), and most patients who survive require ventilators or respiratory assistance for months or even indefinitely. Overall, recovery timing and success varies greatly between patients, while consistently proving to be difficult and a long-term process (Sevjar <i>et al.</i> 2008). | ||
<br>Some unusual cases, such as the onset of acute flaccid paralysis in children under the age of 10, are being analyzed in order to further understand this neurologic illness (Thabet <i>et al.</i> 2013). | <br>Some unusual cases, such as the onset of acute flaccid paralysis in children under the age of 10, are being analyzed in order to further understand this neurologic illness as well as the risk it poses to children (Thabet <i>et al.</i> 2013). | ||
==Future== | ==Future== | ||
| Line 65: | Line 66: | ||
<br><b>Prevention</b> | <br><b>Prevention</b> | ||
<br>The most common and successful methods for outbreak prevention are based on avoiding contact with infected mosquitoes, thereby reducing the risk of infection. Professionals recommend taking any and all precautions to limit the chance of mosquito bite, particularly if West Nile virus has been found in the area. Insect repellents and insect-proofing houses and sitting areas, especially in the summer, are the primary method to protect the public from mosquito bites (Nasci et al. 2005) | <br>The most common and successful methods for outbreak prevention are based on avoiding contact with infected mosquitoes, thereby reducing the risk of infection. Professionals recommend taking any and all precautions to limit the chance of mosquito bite, particularly if West Nile virus has been found in the area. Insect repellents and insect-proofing houses and sitting areas, especially in the summer, are the primary method to protect the public from mosquito bites (Nasci <i>et al.</i> 2005) | ||
<br>A key factor in limiting human risk is surveillance of vectors and any factors that contribute to large-scale outbreaks. Specifically, by studying enzootic and epizootic transmission of West Nile virus in the vectors and hosts, researchers can predict patterns of outbreaks based on viral activity in mosquitoes and take the necessary precautions in the most high-risk areas. | <br>A key factor in limiting human risk is surveillance of vectors and any factors that contribute to large-scale outbreaks. Specifically, by studying enzootic and epizootic transmission of West Nile virus in the vectors and hosts, researchers can predict patterns of outbreaks based on viral activity in mosquitoes and take the necessary precautions in the most high-risk areas. Consistently high-risk areas should develop long term sustainable integrated vector management programs that are able to sample and track all of the environmental factors contributing to outbreaks. This can also assist with vector control by yielding information about populations of infected mosquitoes as well as other hosts that can be eradicated before they are able to infect humans. This is important particularly because the lag time between infection and the onset of infectious symptoms (if any arise) can last up to several weeks, when it is too late to prevent an outbreak. Furthermore, as discussed above, the vast majority of cases are asymptomatic, which yields no warning whatsoever (Nasci <i>et al.</i> 2005). | ||
<br><b>Vaccination</b> | <br><b>Vaccination</b> | ||
<br>Recent epidemics of West Nile virus from 2002-2003 and again in 2012 emphasize the importance of vaccination development, particularly because these epidemics resulted in many fatalities due to neuroinvasive disease. Researchers are currently working to develop a vaccine, and several vaccines are in Phase I and Phase II of clinical trials. Numerous strategies are being employed, including recombinant/subunit vaccines, nucleic acid vaccines, and recombinant virus vaccines (Iyer et al. 2013). However, as of yet, no successful human vaccine has been formulated. | <br>Recent epidemics of West Nile virus from 2002-2003 and again in 2012 emphasize the importance of vaccination development, particularly because these epidemics resulted in many fatalities due to neuroinvasive disease. Researchers are currently working to develop a vaccine, and several vaccines are in Phase I and Phase II of clinical trials. Numerous strategies are being employed, including recombinant/subunit vaccines, nucleic acid vaccines, and recombinant virus vaccines (Iyer <i>et al.</i> 2013). However, as of yet, no successful human vaccine has been formulated. | ||
==Conclusions== | ==Conclusions== | ||
| Line 76: | Line 77: | ||
==References== | ==References== | ||
<br>1. Burton J.M., Kern R.Z., Halliday W., Mikulis D., Brunton J., Fearon M., Pepperell C., Jaigobin C. Neurological manifestations of West Nile virus infection. Can. J. Neurol. Sci.2004 | <br>1. Burton J.M., Kern R.Z., Halliday W., Mikulis D., Brunton J., Fearon M., Pepperell C., Jaigobin C. Neurological manifestations of West Nile virus infection. Can. J. Neurol. Sci.2004 | ||
<2. Busch, M.P., S.H. Kleinman, L.H. Tobler, H.T. Kamel, P.J. Norris, I. Walsh, J.L. Matud, H.E. Prince, R.S. Lanciotti, D.J. Wright, J.M. Linnen, and S. Caglioti. 2008. Virus and Antibody Dynamics in Acute West Nile Virus Infection. The Journal of Infectious Diseases. 198:1-10. | <br>2. Busch, M.P., S.H. Kleinman, L.H. Tobler, H.T. Kamel, P.J. Norris, I. Walsh, J.L. Matud, H.E. Prince, R.S. Lanciotti, D.J. Wright, J.M. Linnen, and S. Caglioti. 2008. Virus and Antibody Dynamics in Acute West Nile Virus Infection. The Journal of Infectious Diseases. 198:1-10. | ||
<br>3. Centers for Disease Control and Prevention. 2014 “Flaviviridae.” Viral Special Pathogens Branch. | <br>3. Centers for Disease Control and Prevention. 2014 “Flaviviridae.” Viral Special Pathogens Branch. | ||
<br>4. Centers for Disease Control and Prevention. 2014 “West Nile Virus.” Division of Vector Borne Diseases. | <br>4. Centers for Disease Control and Prevention. 2014 “West Nile Virus.” Division of Vector Borne Diseases. | ||
Latest revision as of 15:35, 2 October 2015
Background
By Kate Lang
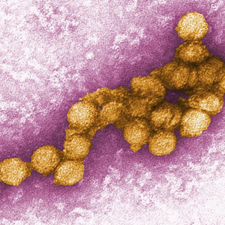
Viruses are widespread and diverse, are transmitted via many vectors, and cause a variety of illnesses. Many dangerous viruses are transmitted via insects (arthropods) such as mosquitoes. The most common arbovirus (arthropod-borne virus) worldwide is West Nile virus (WNV). Recent epidemics have brought this virus into the global spotlight as an emerging threat requiring immediate attention for future prevention (Campbell et al. 2002).
West Nile virus (Flavividae) is a member of the family Flavivirus and is in the same serocomplex (antigen group) as Japanese encephalitis virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus (Sejvar et al. 2006). Other well-known flaviviruses from this family include Yellow Fever and Dengue Fever, which are also mosquito-borne pathogens (CDC, Flavivirus). West Nile virus was first introduced in the United States in New York in 1999, and has since spread all across the lower 48 states, becoming endemic. Cases of West Nile virus are particularly concentrated in the Midwest and Rocky Mountain states during the mid to late summer; however infections have appeared all across the country (Nasci et al. 2013).
Patients infected with West Nile develop a wide range of symptoms, though in most cases no symptoms or only very mild symptoms occur. Of those infected with West Nile virus, 70-80% are asymptomatic and approximately 25% develop West Nile fever, which results in a wide spectrum of relatively minor symptoms (Sejvar et al. 2006). However, in rare cases (less than 1%) certain people develop severe neurologic disease, which includes encephalitis, meningitis, and acute flaccid paralysis (Burton et al. 2004). These neurologic disorders tend to be very severe and carry a relatively high fatality rate of 10% (Sejvar et al. 2005).
Current research involving West Nile virus focuses on preventing future epidemics as well as the development of a vaccine. In the meantime, efforts to survey outbreaks as well as limit human exposure are utilized to prevent possible outbreaks of the disease. Emerging research also continues to explore the often fatal neuroinvasive infections due to West Nile virus in the attempts to prevent and treat these as well.
Structure and Genome
West Nile virus is a spherical virus that possesses a membrane-enclosed nucelocapsid core and is typically between 40 and 50nm in diameter. The West Nile virions are icosahedral, meaning the genome is contained in a 20-face polyhedron capsid (Iyer et al. 2013). The genome is positive single stranded RNA that is approximately 11kb in size. As a member of Group IV of the Baltimore classification system of viruses, West Nile virus is made up of a positive sense-coding strand that can be directly translated into one viral polyprotein. This virus does not have the ability to replicate RNA directly however, and must rely on other RNA polymerases to complete this task. West Nile virus contains codes for three structural proteins, including proteins that make up the envelope, membrane, and capsid, as well as seven proteins that are unrelated to the structure (Sejvar et al. 2005). The envelope protein (E) is particularly interesting and important for viral transmission because it acts as a surface protein that allows for attachment onto host cells, subsequent membrane penetration, fusion, and assembly of West Nile virions. Without this protein the virus would not be able to insert into host cells and complete replication (Sejvar et al. 2005).
Viral Life Cycle
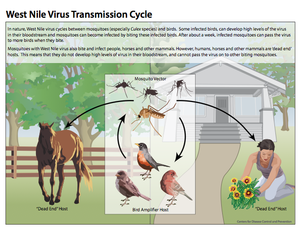
West Nile virus typically cycles between birds and mosquitoes, with the birds acting as the amplifier host and the mosquitoes acting as the vectors (Figure 2) (Girard et al. 2005). Studies have shown that the virus is maintained in mosquitoes during the winter months, after which, the enzootic cycle begins. This occurs when mosquitoes carrying West Nile virus feed on birds and transmit the disease to the avian hosts. Infected birds generate high levels of infection, known as high titer viremia, which allows for the virus to be passed to uninfected mosquitoes once they feed on these birds (Girard et al. 2005). Infected mosquitoes continue to pass the virus on to every susceptible organism they come into contact with and feed on. Ultimately, once the prevalence of infected avian hosts and mosquitoes in a certain area reaches a high enough level, mosquitoes that primarily feed on mammals and other organisms besides birds become infected as well. It is via these mosquito species that humans and other large mammals become infected (Nasci et al. 2013). However, unlike infected birds, humans and other organisms such as horses are dead-end hosts. These hosts do not generate high enough viral loads, therefore uninfected mosquitoes cannot acquire the virus from these hosts and the cycle hits a "dead end" (Nasci et al. 2013).
Transmission to Human Hosts

Vectors
Mosquitoes, particularly members of the Culex family, act as the primary vectors of West Nile virus. The most common mosquito vectors are Culex pipiens pipiens, Culex pipiens quinquefasciatus, and Culex tarsalis (Figure 3)(CDC, West Nile Virus). However, at least 58 species of mosquitoes have been identified as major vectors for West Nile virus in North America (Sevjay et al. 2006). The staggering number of mosquito populations covering the contiguous United States allows for easy transmission of West Nile virus in numerous locations across the country.
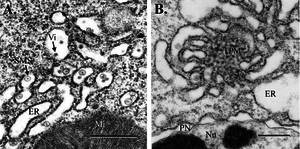
The vector mosquitoes become infected with the virus after feeding on host animals, such as birds, that are carrying the virus. Three days after the infected blood is ingested by the mosquito, the virus begins to replicate in the mosquitoes’ midgut epithelial cells-particularly in the rough endoplasmic reticulum cisternae. The virus then escapes through the basal lamina, particularly in the fatty regions, and is not found in the muscle tissue until much later (Girard et al. 2005). Infection of the midgut results in several morphological changes in the midgut, such as membrane proliferation and swelling of the cisternae (Figure 4). The most notable change, however, is the concentration of smooth membrane structures in the lumen of the rough endoplasmic reticulum. By 21 days post infection, high levels of West Nile virus replication can be detected in the midgut muscle, and vacuoles containing high levels of virions are expelled (Girard et al. 2005). Viral replication occurs in other areas of the mosquito as well, particularly the brain and salivary glands. However, replication consistently occurs primarily in the fatty cells no matter the location in the mosquito. After the virus has replicated inside the mosquito it can be passed to birds or to dead end hosts such as humans once the mosquito feeds on these new organisms (Nasci et al. 2013).
Non-Vector Transmission
Although the majority of West Nile virus cases are transmitted via mosquito vectors, there are alternative ways in which humans acquire the disease. The most common mechanism of transmission, besides mosquitoes, is human-to-human transmission via contaminated blood products or infected tissues.
Blood transfusions of contaminated or previously infected blood have led to several new cases of West Nile virus. Infections resulting from this type of transmission have subsequently led to an increase in screening of all blood donations and transfusions to curtail the spread of the virus. However, despite the increase in surveillance of blood, occasional cases still occur, which indicates that some blood may contain low enough levels of virons as to escape detection, yet still be capable of causing disease (Sejvar et al. 2005). Similarly, transplantation of contaminated organs or tissues have led to the spread of the virus in new patients. Patients who become infected as a result of a contaminated transplant tend to have a higher chance of acquiring dangerous neuroinvasive disease, therefore screening of any and all transplanted tissues is crucial to decrease patients’ risk (Sejvar, 2014). Future developments in testing, specifically those that are able to detect lower viral loads in blood and tissue samples, will be essential in preventing the transmission of West Nile virus via these pathways (Busch et al. 2008).
Another mode of transmission occurs between infected pregnant women and their fetus. The virus can be transmitted to the fetus through the placenta, resulting in the infection of the infant and, subsequently, a multitude of neurological birth defects. There is also evidence that contaminated breast milk can transfer the virus to infants (Nasci et al. 2005).
Susceptible Hosts
Currently, evidence suggests that all humans are potential dead end hosts for West Nile virus. Location and time of year play a significant role in the threat of infection, however the most crucial factor is the proximity to vectors and the time spent exposed to vectors. People who work outside in mosquito-heavy areas are at a higher risk for infection of the virus. Furthermore, those who live in the Midwest or Rocky Mountain States should avoid dense mosquito populations or protect themselves with repellent and protective clothing (Nasci et al. 2005). Horses and other large mammals are also susceptible, though less is known about how the virus affects these animals.
The most important factor in determining host susceptibility is age. Older age, in particular, plays a key role in predicting the probability of the onset of neuroinvasive diseases. Studies have shown that the probability of patients acquiring neuroinvasive diseases increases 150% with every 10 years of age (Sejvar, 2014).
Pathology

The dynamics of West Nile virus infection in the human body have been the topic of recent studies. The work completed by Busch et al. 2008 explored the early stages of infection in order to improve early detection as well as prevention techniques. In doing so, these researchers also gained an in depth understanding of the activity of the virus in the body and the body's response. In this experiment, 245 donors were sampled over the course of a six month period for West Nile virus viral load and antibody production. Using these data, Busch and colleagues were able to generate an accurate model of viral dynamics, including the amount of time it took the body to generate an immune response (as indicated by the production of West Nile virus-specific IgM and IgG antibodies (Figure 5). They were able to detect lower levels of viremia after less than a day post-exposure, as well as show that antibodies are typically generated in the body after approximately 4-7 days- IgM seroconversion occurs before IgG. Further work analyzing the body's response to infection by West Nile virus is important, as there is still much that is unknown.
After infection with West Nile virus, it takes approximately two to four days before viremia levels peak in human hosts. Currently the invasion mechanism utilized by the virus for entry into the Central Nervous System is unknown, however researchers speculate the virus is able to invade cerebral endothelial cells before migrating throughout the brain. After the virus has propagated throughout the body, symptoms will begin to manifest in 25% of cases (Sejvar, 2014).
Symptoms
The majority of people who are infected with West Nile virus are asymptomatic (approximately 70-80% of cases). In these cases the infected persons are likely completely unaware they were even exposed to the virus (Sejvar, 2014).
In approximately 20-25% of cases, patients develop febrile illness and a variety of accompanying symptoms. This is known as West Nile fever and it has a typical incubation period of 2-14 days before the development of symptoms. The symptoms of West Nile fever are body aches (myalgias) and joint pains, as well as headaches, vomiting, diarrhea and a rash. The appearance of a rash has been found more frequently in younger patients, which leads medical researchers to question whether host or immune response dictates the probability of a rash developing (Sejvar, 2014). Patients also experience a relatively rapid onset of fever. Overall, these symptoms can range from mild to more severe, however they typically disappear after a few weeks. In some cases, patients will suffer from prolonged fatigue for months after any other symptoms have disappeared. Treatment for the West Nile febrile illness is limited to over-the-counter pain relievers and any other mild analgesics that temporarily relieve basic symptoms and pain. Though there is technically no cure, most patients make a complete recovery after a few months. Death resulting from West Nile fever mainly threatens elderly patients or those who are already immunocompromised (Sejvar, 2014).
In rare cases, less than 1%, patients will develop severe symptoms as a result of dangerous neurological illnesses. These neuroinvasive diseases include encephalitis (inflammation of the brain), aseptic meningitis (inflammation of the spinal cord and surrounding tissue), and acute flaccid paralysis (Frih-Ayed et al. 2005). Patients suffering from neurologic infection can exhibit a variety of symptoms, including headaches, seizures, and altered mental status, such as coma. This can be particularly dangerous for the elderly as well as those living with other medical problems, such as cancers and cardiopulmonary diseases. Patients must be treated on a case-by-case basis to alleviate symptoms, and hospitalization is commonly required for those suffering from the most severe symptoms and neurological infections. These potential neuroinvasive diseases that result from West Nile virus pose a serious risk, and the observed prevalence of these diseases has continued to mount in the past decade (Sejvar, 2014). Acute flaccid paralysis, which is emerging as a focal point of research, is explored in depth below.
Acute Flaccid Paralysis
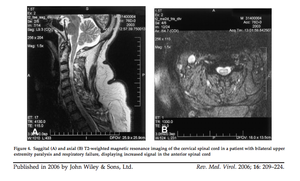
Among the neuroinvasive diseases associated with West Nile virus, acute flaccid paralysis is one of the least well studied, however it is emerging as a serious problem for those afflicted with the virus. Acute flaccid paralysis, or “West Nile poliomyelitis,” refers to the relatively sudden and asymmetrical loss of control and function of localized areas of the body due to infection by the West Nile virus. Recent research suggests that this is the result of viral infection of anterior horn cells, which are motor neurons located in the lower spinal cord (Sejvar et al. 2005). Lab-induced infectious interference of these cells has been shown to cause symptoms identical to the paralysis seen resulting in acute flaccid paralysis (Figure 6). Due to the fact that this manifestation is so rare, it is often confused with Guillan-Barré syndrome and poliovirus. Additionally, a prominent symptom of West Nile fever is fatigue, however the severity of the weakness from acute flaccid paralysis clearly differentiate it from the fatigue commonly associated with West Nile fever (Figure 7)(Sejvar, 2014). Several recent studies have examined specific cases of acute flaccid paralysis in order to gain a better understanding of what causes the symptoms, as well as how to control and prevent this paralysis in the future.
Onset of paralysis symptoms occurs quickly post-infection- typically between 24 and 48 hours (Sevjar et al. 2006). The paralysis tends to be asymmetrical, and most patients lose function or experience weakness in one limb, though some will experience it in multiple limbs. Changes in facial sensation as well as bilateral facial weakness are common. Perhaps the most dangerous symptom that accompanies acute flaccid paralysis is the disruption of respiratory function as a result of the paralysis of diaphragmatic and intercostal muscles (Sevjar, 2014).
The work completed by Saad et al. examined 3 individual case studies of acute flaccid paralysis and West Nile virus as well as accumulated data on 56 other cases of West Nile virus-induced acute flaccid paralysis. They found that 86% of cases of acute flaccid paralysis due to West Nile virus occurred in people over the age of 40, and 35 out of the 56 people presented with a wide variety of other underlying diseases or health problems, including COPD, previous organ transplants, and cancer. A large majority of patients reported fevers (92%) whereas only 17% presented with nuchal rigidity (menigismus). A surprising number of patients experienced weakness in all four limbs (56%), and overall there was a mortality rate of 22%, predominately as a result of respiratory failure.
Overall, current data suggest that patients who acquire acute flaccid paralysis as a result of West Nile virus undergo a difficult and extensive recovery period that is rarely completely successful. Motor control and strength recovery in effected limbs varies between patients, and physical therapy is typically required for a long period of time after infection. The mortality rate of acute flaccid paralysis is relatively high, particularly for elderly and immunocompromised individuals. The main cause of mortality is respiratory failure (50% of deaths are due to acute neuromuscular respiratory failure), and most patients who survive require ventilators or respiratory assistance for months or even indefinitely. Overall, recovery timing and success varies greatly between patients, while consistently proving to be difficult and a long-term process (Sevjar et al. 2008).
Some unusual cases, such as the onset of acute flaccid paralysis in children under the age of 10, are being analyzed in order to further understand this neurologic illness as well as the risk it poses to children (Thabet et al. 2013).
Future
Currently there is no vaccine or antiviral treatment for West Nile virus infection. As a result, other methods must be utilized in order to prevent and control outbreaks as well as limit exposure and support those who become infected.
Prevention
The most common and successful methods for outbreak prevention are based on avoiding contact with infected mosquitoes, thereby reducing the risk of infection. Professionals recommend taking any and all precautions to limit the chance of mosquito bite, particularly if West Nile virus has been found in the area. Insect repellents and insect-proofing houses and sitting areas, especially in the summer, are the primary method to protect the public from mosquito bites (Nasci et al. 2005)
A key factor in limiting human risk is surveillance of vectors and any factors that contribute to large-scale outbreaks. Specifically, by studying enzootic and epizootic transmission of West Nile virus in the vectors and hosts, researchers can predict patterns of outbreaks based on viral activity in mosquitoes and take the necessary precautions in the most high-risk areas. Consistently high-risk areas should develop long term sustainable integrated vector management programs that are able to sample and track all of the environmental factors contributing to outbreaks. This can also assist with vector control by yielding information about populations of infected mosquitoes as well as other hosts that can be eradicated before they are able to infect humans. This is important particularly because the lag time between infection and the onset of infectious symptoms (if any arise) can last up to several weeks, when it is too late to prevent an outbreak. Furthermore, as discussed above, the vast majority of cases are asymptomatic, which yields no warning whatsoever (Nasci et al. 2005).
Vaccination
Recent epidemics of West Nile virus from 2002-2003 and again in 2012 emphasize the importance of vaccination development, particularly because these epidemics resulted in many fatalities due to neuroinvasive disease. Researchers are currently working to develop a vaccine, and several vaccines are in Phase I and Phase II of clinical trials. Numerous strategies are being employed, including recombinant/subunit vaccines, nucleic acid vaccines, and recombinant virus vaccines (Iyer et al. 2013). However, as of yet, no successful human vaccine has been formulated.
Conclusions
West Nile virus currently poses a significant threat to human populations across America as well as the entire world. Vector habitats appear to be increasing, particularly with increasing global temperatures sustaining larger mosquito populations, therefore new epidemics of West Nile virus could be looming. It is crucial to continue research on the virus and the resulting neuroinvasive diseases in order to prevent massive illness and mortality in the future.
References
1. Burton J.M., Kern R.Z., Halliday W., Mikulis D., Brunton J., Fearon M., Pepperell C., Jaigobin C. Neurological manifestations of West Nile virus infection. Can. J. Neurol. Sci.2004
2. Busch, M.P., S.H. Kleinman, L.H. Tobler, H.T. Kamel, P.J. Norris, I. Walsh, J.L. Matud, H.E. Prince, R.S. Lanciotti, D.J. Wright, J.M. Linnen, and S. Caglioti. 2008. Virus and Antibody Dynamics in Acute West Nile Virus Infection. The Journal of Infectious Diseases. 198:1-10.
3. Centers for Disease Control and Prevention. 2014 “Flaviviridae.” Viral Special Pathogens Branch.
4. Centers for Disease Control and Prevention. 2014 “West Nile Virus.” Division of Vector Borne Diseases.
5. Campbell G.L., Marfin A.A., Lanciotti R.S., Gubler D.J. West Nile virus. Lancet Infect. Dis. 2002;2:519–529
6. Frih-Ayed M, Boughammoura-Bouatay A, Ben Romdhane F, Chebel S, Chakroun M, Bouzouia N. 2005. “Acute Flaccid Paralysis of the Upper Limbs Associated with West Nile Virus Infection.” Eur Neurol. 2005; 54(3):159-60.
7. Girard, Y.A., V. Popov, J. Wen, V. Han, S. Higgs. 2005. “Ultrastructural Study of West Nile Virus Pathogenesis in Culex pipiens quinquefasciatus(Diptera: Culicidae).” Journal of Medical Entomology 42(3):429-444.
8. Iyer, A., K. Kousoulas. 2013. “A Review of Vaccine Approaches for West Nile Virus.” Environmental Research in Public Health. 4200-4223.
9. Khromykh, A.A., H. Meka, K.J., Guyatt, E.G. Westaway. 2001. “Essential Role of Cyclization Sequences in Flavivirus RNA Replication.” J. Virol. vol. 75 no. 146719-6728.
10. Nasci, R.S., M. Fischer, N.P. Lindsey, R.S. Lanciotti, H. M. Savage, N. Komar, J. C. McAllister, J. Mutebi, J. M. Lavelle, E. Zielinski-Guitierrez, L.R. Peterson. 2013. “West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control.” Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. pp1-67.
11. Saad, M., S. Youssef, D. Kirschke, M. Shubair, D. Haddadin, J. Myers. 2005. “Acute flaccid paralysis: the spectrum of a newly recognized complication of West Nile virus infection. Journal of Infection. Vol. 5 Issue 2. Pp 120-127.
12. Sejvar J.J. The long-term outcomes of human West Nile virus infection. Clin. Infect. Dis.2007;44:1617–1624
13. Sejvar, JJ and A.A. Marfin. 2006. “Manifestations of West Nile neuroinvasive disease.” Rev. Med. Virol.; 16: 209–224.
14. Sejvar J.J., Curns A.T., Welburg L., Jones J.F., Lundgren L.M., Capuron L., Pape J., Reeves W.C., Campbel G.L. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J. Neuropsychol. 2008;2:477–499.
15. Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Ewing D, Mazowiecki M, et al. 2005. “West Nile virus-associated flaccid paralysis.” Emerg Infect Dis.;11:1021–7.
16. Sejvar J.J., Bode A.V., Marfin A.A., Campbell G.L., Pape J., Biggerstaff B.J., Petersen L.R. West Nile Virus-associated flaccid paralysis outcome. Emerg. Infect. Dis. 2006;12:514–516.
17. Thabet, F.I., S.E. Servinsky, F. Naz, T.E. Kovas, T. O. Raghib. 2013. “Unusual Case of
West Nile Virus Flaccid Paralysis in a 10-Year-Old Child. Pediatric Neurology. Volume 48, Issue 5, May 2013, Pages 393–396.
