Xanthomonas citri
Classification
Higher order taxa
Bacteria; Proteobacteria; Gammaproteobacteria; Xanthomonadales; Xanthomonadanceae
Species
Xanthomonas citri
|
NCBI: Taxonomy |
Description and significance
X. citri is part of the Xanthomonas genus of phytopathogenic bacteria that inhabit the phyllosphere of citrus plants species and crops and produce the citrus canker disease. The bacteria is named for their pathogenic behaviors that target citrus fruits and crops. At least three forms of the disease exists, with one of them, Canker A, being the most destructive form.
Although the geographical origin and the original discoverer of X. citri is debatable; Lee (1918) stated that the species originated from southern China, however, Fawcett and Jenkins (1933) reported that the species originated from parts of India and Java. Fawcett and Jenkins’ findings suggest that X. citri originated from tropical areas in Asia. X. citri was first described in 1915, after it was found in the Gulf States of USA [3]. Severe infectious forms of the disease can cause premature fruit drop, defoliation, blemished fruit, and reduced fruit quality. In addition, warm, humid, or cloudy climate combined with heavy rainfall and strong wind exacerbates the impacts of the disease.
X. citri can also produce biofilms on both biotic and abiotic surfaces of organisms, particularly on cankers of diseased plants. The bacteria utilize flagella-mediated motility throughout biofilm formation, and sliding and swimming motility (without flagella) through diffusible signal factor (DSF) [5]. In addition, X. citri utilizes type IV pilus for twitching motility, biofilm development, and adherence [4]. X. citri secretes proteins through the type III secretory system into the extracellular matrix [6]. The bacteria are Gram-negative, rod-shaped, aerobic [2], and nonsporeforming, and also produces yellow, water-insoluble pigments due to the presence of carotenoid nostoxanthin and oxidatively metabolizes sugars [1].
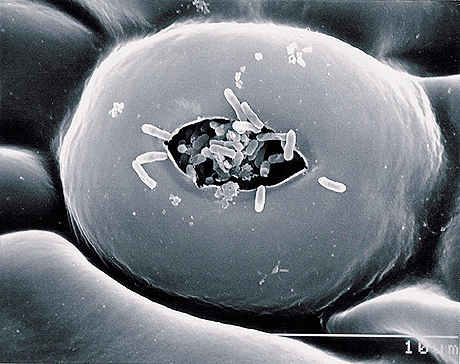
SEM of stomata on grapefruit leaf with Xac bacteria entering stomatal chamber, from The American Phytopathological Society.http://www.apsnet.org/publications/apsnetfeatures/Pages/citruscanker.aspx
Nutrition and growth
a.Describe the growth characteristics of your bacterial species; sources of C, N, electrons; respires/ferments, uses O2, etc.
Growth requires methionine or cysteine and is inhibited by 0.02% triphenyltetrazolium chloride. Biovars may be distinguished by utilization of mannitol (http://www.cabi.org/isc/datasheet/56921
Growth develops in 48 to 72 hours and colonies are creamy-yellow (on PDA) to straw-yellow (on NA). The identification of isolated bacteria can be confirmed by pathogenicity to grapefruit leaves or by the polymerase chain reaction (PCR) using specific primers (Goto, 1992).
L-glutamate plays a central role in nitrogen metabolism in all living organisms. In the genus Xanthomonas, the nitrogen nutrition is an important factor involved in the xanthan gum production, an important exopolysaccharide with various industrial and biotechnological applications. In this report, we demonstrate that the use of L-glutamate by the phytopathogen Xanthomonas axonopodis pv. citri as a nitrogen source in defined medium significantly increases the production of xanthan gum. (https://www.ncbi.nlm.nih.gov/pubmed/23719672)
we report the emergence of some natural field strains of Xanthomonas citri subsp. citri (Xcc) capable to use lactose as a sole carbon source to produce xanthan gum. (http://search.proquest.com/docview/1510375273?pq-origsite=gscholar)
This bacterium is exposed to reactive oxygen species (ROS) at different points during its life cycle, including those normally produced by aerobic respiration or upon exposition to ultraviolet (UV) radiation. Moreover, ROS are key components of the host immune response. Among enzymatic ROS-detoxifying mechanisms, catalases eliminate H2O2, avoiding the potential damage caused by this specie. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4807922/)
For laboratory study of ‘’X. Citri,’’ the bacteria may be stored in 4-8°C until processing (https://www.ippc.int/static/media/files/publication/en/2016/01/DP_06_2014_En_2015-12-22_PostCPM10_InkAmReformatted.pdf). For good culture conditions, ‘’X. Citri’’ requires incubation at 28°C in NB liquid medium for 16-20 hours in 5.4 pH. (https://www.researchgate.net/publication/302917518_Mapping_and_validation_of_Xanthomonas_citri_subsp_citri_genes_regulated_by_putative_plant-inducible_promoter_box_PIP-box).
Citrus waste by-product contains approximately 15.2% cellulose, 18.2% hemicellulose, 24.6% pectin, 15-20% soluble sugars, 6.5-8.0% proteins, 3.8% starch, and 3.5% ashes (Enhanced Materials from Nature: Nanocellulose from Citrus Waste Mayra Mariño 1, Lucimara Lopes da Silva 1, Nelson Durán 2 and Ljubica Tasic 1,*).
In addition, enzymes of X. axonopodis pv. citri are very active on citrus waste biomass (Enhanced Materials from Nature: Nanocellulose from Citrus Waste Mayra Mariño 1, Lucimara Lopes da Silva 1, Nelson Durán 2 and Ljubica Tasic 1,*).
Genome and genetics
X. citri belong to the major prokaryotic branch of bacteria (SOURCE Bergey’s). There are a few distinct bacteria that are closely related to X. citri, such as Xanthomonas vesicatoria (SOURCEhttp://onlinelibrary.wiley.com/store/10.1111/nph.12397/asset/nph12397.pdf?v=1&t=j0w0hadh&s=c91259736dfbcaa1fecda18b4d97bd8007ec6bcf) and Xanthomonas campestris pv. malvacearum by DNA-DNA hybridization (SOURCE Egel et al., 1991).
The complete circular genome sequencing of ‘’X. citri’’ strain A^W12879 was determined by using 454 pyrosequencing and Illumina technology (Jalan et al. 2013). The genome contains a chromosome with DNA GC content of 64.71% and genome size of 5,320,000 base pairs, and also contains two plasmids (circular), pXcaw19 with DNA GC content of 63.07% and 18,869 base pairs and pXcaw58 with DNA GC content of 61.85% and 58,317 base pairs. The plasmid pXcaw19 sequence does not have homology with plasmids ‘’X. Citri’’ subsp. ‘’Citri’’ Asiatic strain 306. The plasmid strain pXcaw58, however, has about 35% similarity with pXAC64. (Source https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3656205/)
‘’X. Citri’’, AW12879 has 4,760 annotated CDS, of which 3,457 can be assigned to a COG functional category. The genome has 2 rRNA operons (identified with FgenesB) and 54 tRNA genes (identified with tRNAscan-SE) (SOURCE https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3656205/).
Pathology
Pathology: How does the microbe cause disease as it interacts with the host? Describe any specific toxins or pathways that are used for invading and causing disease in the host. What treatment is used to inhibit or kill the microbe?
‘’X. Citri’’ is best known as causing the disease citrus canker in fruits. There are various mechanisms for the bacterial propagation of this disease. ‘’X. citri’’ grow out of lesions in leaves, stems, and fruit, and if free moisture exists on the lesions, the bacteria oozes out and spreads its infection across the surface of the lesion. The bacteria may also penetrate out through stomatal pores or wounds caused by thorns, insects, and blowing sand, which are aided by wind-driven rain (most common method of penetration). (http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/CitrusCanker.aspx
‘’X. Citri’’ remain alive in the margins of lesions, until the leaves or fruit fall onto the ground. The bacteria also die upon exposure to dry surface after oozing through lesions onto surfaces; accelerated death also occurs as a result of sun exposure. (http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/CitrusCanker.aspx)
Sanitation, windbreaks, and leafminer control with frequest copper spray application have been considered plausible and important methods of treatment of plants and trees infected by ‘’X. Citri.’’ It has been suggested that several weeks of copper spray application may decrease the infection, and windbreaks may greatly reduce the speed of bacterial infection spread and severity and also enhance the copper spray’s impact. Leafminer control may be decrease infection in young trees and certain cultivars (http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/CitrusCanker.aspx)
X. citri infected fruit, foliage, and stems from The American Phytopathological Society. http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/CitrusCanker.aspx
X. citri leaf lesions start as pinpoint spots and attain a maximum size of 2 to 10 mm diameter, from The American Phytopathological Society.http://www.apsnet.org/edcenter/intropp/lessons/prokaryotes/Pages/CitrusCanker.aspx
Current Research
Foliar Application of Biofilm
In one recent study, scientists framed their research based on the idea that biofilm formation is important during early infection of X. citri on host leaves. They focused on testing their hypothesis that small molecules that inhibit biofilm formation of X. citri would enhance control of the citrus disease. Through their investigation and study, they found that D-leucine (amino acid involved in energy and metabolism) and 3-indolyl acetonitrile (known for inhibiting biofilm substance on Escherichia coli and Pseudomonas aeruginosa) inhibited biofilm formation of X. citri on different abiotic surfaces and host leaves. The scientists observed that the biofilm inhibition occurred at a concentration lower than their set minimum inhibitory concentration. In addition, they found that when D-leucine and IAN are applied alone or combined with copper, in greenhouse assays, such combination reduced the number of canker lesions and bacterial populations of X. citri on citrus host leaves. https://www.ncbi.nlm.nih.gov/pubmed/23901828
ASDS
References
[1] Bergey, D. H., Buchanan, R. E., Gibbons, N. E., & American Society for Microbiology. 2005. Bergey's Manual of Systematic Bacteriology: Proteobacteria, 2nd edn. Baltimore (MD): Williams & Wilkins. p. 235-762.
[2] Büttner D., Bonas U. "Regulation and secretion of Xanthomonas virulence factors". "FEMS Micro Rev". March 2010. 34(2): 107-133.
[3] Das A.K. "Citrus canker—A review". J Appl Hort. June 2003. 5(1): 52-60.
[4] Dunger G, Guzzo CR, Andrade MO, Jones JB, Farah CS. "Xanthomonas citri subsp. citri type IV Pilus is required for twitching motility, biofilm development, and adherence". "Mol Plant Micr Int". October 2014. 27(10): 1132-47.
[5] Pablo C. Bogino, María de las Mercedes Oliva, Fernando G. Sorroche, and Walter Giordano. "The Role of Bacterial Biofilms and Surface Components in Plant-Bacterial Associations". "Int J Mol Sci". August 2013. 14(8): 15838–15859.
[6]: Zimaro et al.:."The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation". "BMC Microbiology". April 2014. 14(96): 1-15
Authored by YGJ, a student of CJ Funk at John Brown University