Anti-Helicobacter Pylori Activity From Natural Products
Alternative therapies from natural plants and foods are gaining popularity in order to find products to help combat the drug-resistant strains of Helicobacter pylori. Since H. pylori causes around 85 percent of all duodenal and peptic ulcers, an effective and natural treatment would prove beneficial. Flavonoid extracts from plants have exhibited significant bactericidal activity that affects vital enzymes for the bacteria’s survival. Many studies have examined and found antimicrobial activity in acetone and methanol extracts from Pakistanian, Indian, and Iranian medicinal plants. Additionally, studies have found that fractional isolates of honey and turmeric are responsible for antibacterial activity. These compounds provide new lead molecules that can help eradicate H. pylori and cure most duodenal and peptic ulcers.
H. pylori infection
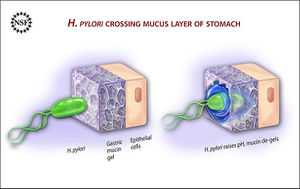
For many individuals, Helicobacter pylori, helix shaped gram-negative bacteria, reside in the stomach that many times lead to duodenal and peptic ulcers. In the outer membrane, H. pylori possesses many proteins: adhesins, porins, iron transporters, and flagellum. This gastric bacterium moves highly by its own flagella. Protecting itself from the acidic stomach, H. pylori uses the flagella to dig into the mucous lining in order to get to the epithelial cells underneath.15 At the epithelial cells underneath, the pH is more neutral, so the H. pylori senses the pH gradient (chemotaxis) and moves closer to these epithelial cells. When food goes through the digestive tract, the bacteria is not swept away with the mucus and food to the duodenum because it is attached to the epithelial cells. The outer membrane of H. pylori produces adhesin proteins, which help bind the bacteria to the lipid and carbohydrates in the epithelial cell membrane.15
In addition to adhering to the epithelial cell membrane, H. pylori can survive in the acidic stomach because it produces urease in order to neutralize the acid. Urease converts urea to ammonia and carbon dioxide. The basic ammonia is able to neutralize the stomach acid. However, this ammonia and other H. pylori produced biochemicals (protease, vacuolating cytotoxin, and phospholipase) are harmful to the gastric epithelial cells. This bacterial colonization inflames the stomach at the infection site. Inflammation causes stomach acid and pepsin, a digestive enzyme, to increase production in order to keep the same gastric environment; this leads to duodenal and stomach ulcers because the increased acid load and pepsin harms the gastric epithelial cells.15 The location of H. pylori infection and ulcers varies depending on the amount of acid individuals produce. For individuals producing copious amounts of acid, the bacteria reside closer to the pyloric antrum, which avoids the acid secreting cells at the entrance of the stomach. Individuals with normal to lower amounts of gastric acid, the H. pylori can exist throughout the stomach.
In order to cure these H. pylori created ulcers, antibiotics are given to inhibit the bacterium. Antibiotics, clarithromycin and amoxicillin, and a proton pump inhibitor are typically used to inhibit H. pylori growth to cure the ulcer.15 This triple therapy for the elimination of H. pylori faces uprising resistance problems demanding new antibiotic candidates such as natural products.
Bactericidal Activity of Plant Extracts
Many different extracts from plants have been shown to have antimicrobial activity. GutGard™ is a flavonoid rich extract of Glycyrrhiza glabra Linn, which is commonly known as Licorice. The company sells this product to those who suffer from gut and digestion related issues. Many studies have been conducted to determine the validity of GutGard™.3,11 Furthermore, different acetone and methanol extracts were taken from traditional medicinal plants to determine the minimal inhibitory concentration on different strains of H. pylori.2,10
Flavonoid Extract
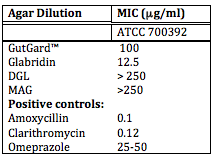
Glycyrrhiza glabra is normally found in the Mediterranean and parts of Asia. Many older civilizations used the root of this plant for its medicinal properties to cure stomach ulcers, kidney stones, skin eruptions, and more. Due to the increasing drug resistant strains of H. pylori, studies are trying to demonstrate and verify the antimicrobial activity of this natural plant. Through a microbroth dilution method, the minimum inhibitory concentrations (MIC) against several variant strains of H. pylori were determined for GutGard™, glabrin, deglycyrrhizainated licorice (DGL), monoammonium glycyrrhizinate (MAG).3 Glabridin, a flavonoid rich extract of GutGard™, appeared to be the active ingredient causing anti-H. pylori activity.3
GutGard’s™ mechanism of action is not quite confirmed, but through an in vitro study, GutGard™ demonstrated a protein synthesis inhibition via the reduction in (35)S methionine into H. pylori ATCC 700392 strain.3 Furthermore, this potential antibiotic exhibited an inhibitory effect on DNA gyrase and dihydrofolate reductase. Ciprofoxacin, levofoxacin, and fluoroquinolones, antibiotics that inhibit DNA gyrase, are being considered as possible drugs for the eradication of Helicobacter pylori.12 Thus, DNA gyrase may play an essential role in the growth and survival of H. pylori. Comparing the inhibition of DNA gyrase of GutGard™ to ciprofloxacin, GutGard™ had an MIC50 of 4.40 μg/ml while the positive control, ciprofloxacin, showed an MIC50 value of 12.31 ng/ml.3 If dihydrofolate reductase is blocked, the bacterial cells die because of its inhibition of DNA synthesis. When compared to the positive control methotrexate, GutGard™ was found to moderately inhibit H. pylori.3
A double blind randomized study was conducted to evaluate the inhibitory effects of GutGard™.11 Participants were randomly placed into the placebo (n=52) and treatment (n=55) groups. Both groups orally received their respective drug once daily for 60 days. A 13C-urea breath test and Stool Antigen test were performed at days 0, 30, and 60 to measure the H. pylori levels in the stomach. Using the analysis of variance, chi-square, and Fisher’s exact probability test to compare the overall treatment conditions, the GutGard™ treatment group showed a statistically significant improvement in the management of H. pylori.11
Even at a low stomach pH, flavonoids still seem to exhibit antibacterial activity. In general, flavonoids tend to have an antimicrobial effect against different H. pylori strains. The antibacterial effects of 7-O-Butylnaringenin, a novel flavonoid synthesized from citrus waste, and various natural flavonoids were studied.9 Hesperetin and naringenin exhibited the most antimicrobial effects for the natural flavonoids.9 However, 7-O-Butylnaringenin exhibited a higher inhibitory effect against urease activity of H. pylori than the natural flavonoids. The morphological changes of H. pylori show that 7-O-buthylnaringen and hesperetin damage the bacterial cell membrane at 200 μM.9 This study shows how different natural products can be slightly modified to yield a compound with greater inhibitory potential.
Acetone and Methanol Extracts
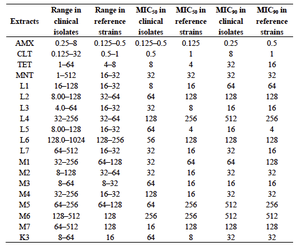
Many plants from the Middle Eastern, Mediterranean, and Indian area have traditionally been used to treat common ailments. Acetone and methanol extracts from these plants possess some antibacterial properties against different strains of H. pylori. Using the agar dilution method, the minimum inhibitory concentrations of acetone, methanol, and aqueous extracts were determined from various plants in the Punjab province of Pakistan.2 In order to compare the efficacy of the plant extracts, the percent of H. pylori inhibition was calculated. The methanol and acetone extracts from Acacia nilotica and Calotropis procera had significant antibacterial activity, but the aqueous extracts did not.2 Furthermore, the acetone extracts were better antimicrobial agents than the methanol extracts; there probably is more active phytochemical components in the acetone extracts. Anti-H. pyori activity of two acetone extracts (L3 & L5) and two methanol extracts (M2 & M3) had better antibiotic activity than metronidazole, and the same as tetracycline.2
H. pylori releases urease in order to convert urea into ammonia. This ammonia protects the bacteria from the acidic stomach, which makes this enzyme vital for the survival of the bacterial. The phenol red method showed that acetone and methanol extracts significantly inhibited urease.2 Using the Lineweaver Burk plots to determine the Michaelis-Menten constants, the mechanism of inhibition for the extracts of Acacia nilotica and Calotropis procera were determined. Extracts from Acacia nilotica exhibited non-competitive inhibition, while the other plant extracts involved a mixed inhibition.2
Combretum molle from South Africa has been widely used to treat gastric ulcers. The agar diffusion test showed the MIC of five different solvent extracts: acetone, ethanol, water, crude extract, and ethyl acetate.10 All the solvent extracts demonstrated some anti-H. pylori activity; however, the acetone extract was highly bactericidal. At MIC90, the acetone extract exhibited inhibition at 2.5 to 5.0 mg/ml depending on the strain of H. pylori.10
Bactericidal Activity of Natural Foods
Honey
Honey’s high acidity, high osmotic effect, concentration of hydrogen peroxide, and various photochemicals all help honey inhibit or kill different bacteria. Additionally, Krishna verified that the acidic medium of the stomach helps augment the antibacterial activity of honey.7 In the n-hexane extract of Goldcrest honey, TLC chromatography found four different column fractions – GCCL, GCF2, GCF3, and CGF4 – to exhibit anti-H. pylori activity.7 Using the broth microdilution method, the column fractions had MIC50 that ranged from 5-10 mg/ml, which showed that there were probably different antimicrobial isolates in each of the fractions. GCF3 exhibited the best antimicrobial activity against various strains of H. pylori.7 The acetic acid, proponoic acid, and flavonoids components all help give honey its antimicrobial activity. In conclusion, the different actions of the volatile compounds present cause the antimicrobial activity.
Another study tested six different local South African honeys and their solvent extracts for anti-H. pylori activity.8 The broth microdilution method determined the MIC50 of the two most active extracts from the six local honeys. All of the honeys had different inhibiting zones, but the Pure Honey and Champagne Royal Train had zone diameters that were not significantly different from amoxicillin, the positive control.8 The chloroform extract in pure honey had the most inhibiting effect from 42 to 72 hours. Similar to the 7-O- butylnaringenin, these honey extracts can serve as a start to make new molecules that are better antimicrobials.8
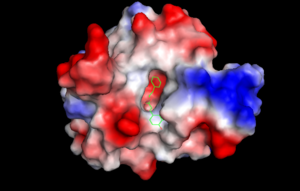
Furthermore, other components from honey, propolis, has been found to exhibit anti-H. pylori activity.4 An essential enzyme, H. pylori peptide deformylase (HpPDF), facilitates the removal of the formal group from the N-terminal of nascent polypeptide chains for newly synthesized proteins. Caffeic acid phenethyl ester (CAPE), an active component of propolis, competitively inhibits HpPDF by blocking substrate entrance. The MIC50 value of CAPE is 4.02 μM.4 The phenyl head of CAPE binds to the hydrophobic active site, while the CAPE tail protrudes to the entrance of the pocket. Hydrogen bonds are also formed with the CAPE and the surrounding amino acids, which exhibit pi-pi interaction. Most PDF inhibitors are pseudopeptides, but CAPE has a different structure. Compared to other PDF inhibitors, no chelation or disruption of metal-dependent catalysis occurs with the HpPDF-CAPE complex at the active site; this actually may lead to less harmful effects on the body.
Turmeric
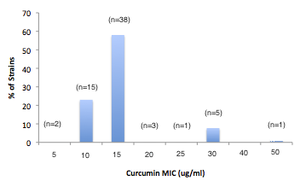
Diferuloylmethane from turmeric, curcumin, has been shown to inhibit H. pylori growth. The antimicrobial activity was examined against 65 different clinical isolates of H. pylori. 5 Depending on the strain, the MIC of curcumin ranged from 5 μg/ml to 50 μg/ml. Looking at kinetic data, curcumin seems to act as a non-competitive inhibitor in order to hinder the shikimate dehydrogenase (SHD) activity. SDH catalyzes the reduction of 3-dehydroshikimate to shikimate in the shikimate pathway.6 The difference in MIC values suggest that this enzyme may not always be essential for the bacteria’s survival.5 Furthermore, the effects of curcumin on a H. pylori infected mice was studied histologically. This test showed that curcumin was highly effective in treating H. pylori in the mice’s stomach and restoring the gastric damage. Curcumin serves potential as an alternative therapy because it has shown antimicrobial and mucoadhesive properties. The mucoadhesive microspheres of curcumin can serve as a drug to treat H. pylori in combination with other antimicrobials.1
Further Reading
There are several other natural products that may have anti-H. pylori activity. Broccoli, garlic, red wine, and green tea have also been accredited to having some bactericidal effects against H. pylori, but the MIC for these products have not been determined.14 Even though these natural products may have some antimicrobial effects, they still are not as efficient as other known antibiotics. However, these natural products serve as an example to find other lead molecules that serve as better antibiotics for most strains of H. pylori. These new therapies may help combat the many side effects from the known antibiotics and make treating H. pylori more effective.
[1]—Alternative treatments for Helicobacter pylori infection
[2]- Protocol to eradicate all H. pylori infections in the stomach
[3]- Honey as an antimicrobial
References
1. Ali, Sajid, Vinay Pandit, Mahendra Jain, and Kanhiya L. Dhar. "Mucoadhesive Microparticulate Drug Delivery System of Curcumin against Helicobacter Pylori Infection: Design, Development and Optimization." Journal of Advanced Pharmaceutical Technology and Research 5.1 (2014): 48-56.
2. Amin, Muhammad, Farooq Anwar, Fauqia Naz, Tahir Mehmood, and Nazamid Saari. "Anti-Helicobacter Pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants." Molecules 18.2 (2013): 2135-149.
3. Asha, Mannanthendil Kumaran, Debnath Debraj, D'souza Prashanth, Jothie Richard Edwin, H.s. Srikanth, Nithyanantham Muruganantham, Shekhar Michael Dethe, Bhaskar Anirban, Balachandran Jaya, Mundkinajeddu Deepak, and Amit Agarwal. "In Vitro Anti-Helicobacter Pylori Activity of a Flavonoid Rich Extract of Glycyrrhiza Glabra and Its Probable Mechanisms of Action." Journal of Ethnopharmacology 145.2 (2013): 581-86.
4. Cui, Kunqiang, Weiqiang Lu, Lili Zhu, Xu Shen, and Jin Huang. "Caffeic Acid Phenethyl Ester (CAPE), an Active Component of Propolis, Inhibits Helicobacter Pylori Peptide Deformylase Activity." Biochemical and Biophysical Research Communications 435.2 (2013): 289-94.
5. De, R., P. Kundu, S. Swarnakar, T. Ramamurthy, A. Chowdhury, G. B. Nair, and A. K. Mukhopadhyay. "Antimicrobial Activity of Curcumin against Helicobacter Pylori Isolates from India and during Infections in Mice." Antimicrobial Agents and Chemotherapy 53.4 (2009): 1592-597.
6. Han, Cong, Lirui Wang, Kunqian Yu, Lili Chen, Lihong Hu, Kaixian Chen, Hualiang Jiang, and Xu Shen. "Biochemical Characterization and Inhibitor Discovery of Shikimate Dehydrogenase from Helicobacter Pylori." FEBS Journal 273.20 (2006): 4682-692.
7. Manyi-Loh, Christy E., Anna M. Clarke, and Roland N. Ndip. "Detection of Phytoconstituents in Column Fractions of N-Hexane Extract of Goldcrest Honey Exhibiting Anti-Helicobacter Pylori Activity." Archives of Medical Research 43.3 (2012): 197-204.
8. Manyi-Loh, C. E., A. M. Clarke, and NDIP E. Green, RN. "Inhibitory and Bactericidal Activity of Selected South African Honeys and Their Solvent Extracts against Clinical Isolates of Helicobacter Pylori." Pak J Pharm Sci. 26.5 (2013): 897-906.
9. Moon, Sun H., Jae H. Lee, Kee-Tae Kim, Yong-Sun Park, Seung-Yeol Nah, Dong Ahn, and Hyun-Dong Paik. "Antimicrobial Effect of 7-O-Butylnaringenin, a Novel Flavonoid, and Various Natural Flavonoids against Helicobacter Pylori Strains." International Journal of Environmental Research and Public Health 10 (2013): 5459-469.
10. Njume, Collise, Anthony J. Afolayan, Amidou Samie, and Roland N. Ndip. "Inhibitory and Bactericidal Potential of Crude Acetone Extracts ofCombretum Molle (Combretaceae) on Drug-resistant Strains of Helicobacter Pylori." Journal of Health, Population and Nutrition 29.5 (2011).
11. Puram, Sreenivasulu, Hyung C. Suh, Seung Kim, Bharathi Bethapudi, Joshua Joseph, Amit Agarwal, and Venkateswarlu Kudiganti. "Effect of GutGard in the Management of Helicobacter Pylori: A Randomized Double Blind Placebo Controlled Study : Table 3." Hindawi (2013): 1-8.
12. Rimbara, Emiko, Norihisa Noguchi, Takashi Kawai, and Masanori Sasatsu. "Fluoroquinolone Resistance in Helicobacter Pylori: Role of Mutations at Position 87 and 91 of GyrA on the Level of Resistance and Identification of a Resistance Conferring Mutation in GyrB."Helicobacter 17.1 (2012): 36-42.
13. Uthman, Ed. Helicobacter pylori. 2007. Flickr. Web. <http://www.flickr.com/photos/78147607@N00/390307642/in/photolist-AuqS7-AuqS8-9hmf44-9pz3W4-fxzkmp>.
14. Ayala, Guadalupe, Wendy Escobedo-Hinojosa, Carlos Felipe De Las Cruz-Herrera, and Irma Romero. "Exploring Alternative Treatments for Helicobacter Pylori Infection." World Journal of Gastroenterology 20.6 (2014): 1450-469.
15. "Helicobacter Pylori." Wikipedia. Wikimedia Foundation, 04 May 2014. Web.
Edited by Amie Patel, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
