Antimicrobial Effects of Honey
Honey has seen a revival recently in the Western medical field, as it has shown inhibitory activity against a range of detrimental and antibiotic-resistant microbes of infected wounds 1. Honey may be the first recorded medicine, having been documented in the Smith Papyrus of Egypt, which dates to between 2200-2600 BC 2. Since ancient times honey has been renowned for its wound-healing properties 3. With the advent of antibiotics, clinical application of honey has been neglected in modern Western medicine, although it is still used in many cultures 3. The overwhelming use of antibiotics has resulted in widespread resistance, therefore alternative antimicrobial strategies are necessary 3.
Honey has demonstrated potent in vitro activity against antibiotic-resistant bacteria and it has been successfully applied as treatment of chronic wound infections not responding to antibiotic therapy 3 . For example, honey has received attention as an important tool against strains of bacteria such as Methicillin-resistant Staphylococcus aureus 4. No microbial resistance against honey has been observed, making it attractive as a treatment for wound infections 4. Honey possesses several antimicrobial properties and can act via various mechanisms of action. There are many different types of honey from around the world, made from different floral sources with variable mechanisms of action. The antimicrobial potency and medical applications of honey are tremendous as it has demonstrated inhibitory effects against a number of pathogenic bacteria 134.
Mechanisms of Action
Honey prevents microbial growth through the use of hydrogen peroxide (H2O2), methylglyoxal (MGO), bee defensin-1, flavonoids, and a relatively low pH (~3.3) 13. As shown in Figure 1, the different active components in honey have been isolated by neutralizing each one individually and observing the effect on its antimicrobial activity. Not all of the factors listed are present in all types of honey, and these compounds must be tested for and considered for clinical applications 3.
Osmolarity
The high osmolarity of honey can also contribute to the inhibition of growth, although this is true of sugar solutions as well 13. Honey is 70 to 80% sugar and this high percentage causes hypertonic conditions that may lead to lysis of microbial cell walls 5.
H2O2
Hydrogen peroxide is produced by the Apis mellifera (honeybee) glucose oxidase enzyme on dilution of honey, and is produced in low but effective concentrations 36. Due to the slow release of H2O2, there is much less cytotoxic damage to the patient’s cells, providing a better method than applying H2O2 directly to wounds 6.
Methylglyoxal
Methylglyoxal is a compound found in manuka honey that was reported to have an antibacterial property 5. MGO can be converted into its inactive form, S-lactoylglutathione, by glyoxalase I and this was done in an experiment to test the effects of MGO on honey’s bactericidal activity (unprocessed Revamil honey was used in this experiment) 3. Neutralization of MGO or H2O2 alone did not alter bactericidal activity of RS honey, but simultaneous neutralization of MGO and H2O2 in a solution diluted with water to 10% honey reduced the killing of B. subtilis by 4-logs 3. At higher concentrations of honey, the bactericidal activity was not affected by neutralization of MGO and H2O2 3.
Bee defensin-1
Bee defensin-1 is found in honey and is the only cationic bactericidal compound currently identified 3. In dilutions of 20% honey and greater, when H2O2 and MGO were neutralized bactericidal activity was retained 3. When bee defensin-1 was also neutralized, the bactericidal activity was strongly reduced at 20% but was not affected at 30 and 40% honey solutions 3. Bee defensin-1 was previously isolated from royal jelly, the major food source for bee queen larvae and was identified in honeybee hemolymph 3. This peptide is secreted by the hypopharyngeal gland of worker bees into collected nectar along with carbohydrate-metabolizing enzymes and bee defensin-1 presumably contributes to protection of royal jelly and honey against microbial spoilage 3.
Flavonoids
Flavonoids are a group of pigments produced by plants and their presence was suggested to contribute to the antimicrobial properties of honey 1. Actions of flavonoids include direct antibacterial activity, synergism with antibiotics, and suppression of bacterial virulence 5. The direct antibacterial activity of flavonoids may be attributable to several tested mechanisms: cytoplasmic membrane damage (caused by perforation and/or a reduction in membrane fluidity possibly by generating hydrogen peroxide), inhibition of nucleic acid synthesis (caused by topoisomerase inhibition and/or dihydrofolate reductase inhibition), inhibition of energy metabolism (caused by NADH-cytochrome c reductsse inhibition and ATP synthase inhibition), inhibition of cell wall synthesis (caused by D-alanine-D-alanine ligase inhibition) and inhibition of cell membrane synthesis (caused by inhibition of several enzymes) 5. There are 14 classes of flavonoids in total, categorized by their chemical nature and structure 5. Because most studies on the mechanism of action of flavonoids were conducted on only one or two types of flavonoids, it remains unclear as to whether flavonoids have multiple mechanisms of action or flavonoids have a single mechanism that has yet to be convincingly determined 5.
pH
Honey has a low pH primarily due to the conversion of glucose into hydrogen peroxide and gluconic acid by glucose oxidase 3. This low pH might also contribute to the bactericidal activity of honey, demonstrated by the titration of the pH of 10-40% honey solutions from 3.4-3.5 to 7.0 combined with neutralization of other bactericidal factors (H2O2, MGO and bee defensin-1) reduced the bactericidal activity of honey to the same level of a honey-equivalent sugar solution 3.
Effectiveness of Different Types of Honey
Manuka Honey
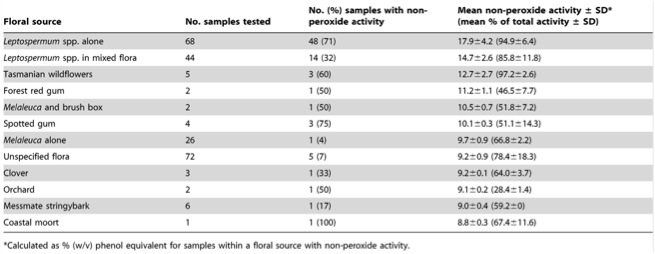
Manuka honey is predominantly harvested in New Zealand and Australia, from bees visiting Leptospermum trees. The minimum inhibitory concentration (MIC) of manuka honey on Stayphylococcus aureus was between 2 and 3% (v/v) ([(volume of solute)/(volume of solution)] x 100%) 4. While manuka honey does release H2O2, its anitmicrobial action also has a phytochemical component 4. In this study by Cooper et al. (1999) the antibacterial activity of manuka and pasture honeys on S. aureus were determined by an agar well diffusion bioassay using phenol as a reference standard antiseptic both in the presence of catalase and not in the presence of catalase, to detect any non-peroxide antibacterial activity; the MIC of each honey was determined by an agar incorporation technique. Manuka honey has received more attention for antimicrobial work recently due to its additional phytochemical compounds.
Pasture Honey
The MIC of honey from a mixed pasture source was between 3 and 4% on S. aureus 4. These honeys prevent growth of S. aureus even when diluted by body fluids a further seven-fold to fourteen-fold beyond the point where their osmolarity ceased to be completely inhibitory 4 Pasture honey acts by releasing H2O2, but often lacks significant amounts of the extra phytochemical components of manuka honey.
One brand of commercial honey called Black Forest honey from Langaneza, Germany was tested and found to inhibit eight different types of microbes at concentrations between 10 to 100% honey solutions7. Growth of all microbes was reduced at 10% honey solutions and completely inhibited at 20% honey solutions for Methicillin-Sensitive S. aureus, Methicillin-Resistant S. aureus, and E. coli, and at 50% honey solutions for P. aeruginosa and C. albicans, and at 100% honey for S. pyogenes, Vancomycin-sensitive enterococci and Vancomycin-resistant enterococci 7.
Medical Honey
Unprocessed Revamil source honey was effective at killing several different strains of bacteria at 10-20% (v/v), while greater than 40% (v/v) of a honey-equivalent sugar solution was required for similar activity 3. Another medical grade honey, Medihoney, comes from honey from Leptospermum flowers, making it very similar to manuka honey. In Medihoney, however, there are numerous steps taken to guarantee the sterility of the honey.
Artificial Honey
In a study on the effect of honey on Streptococcus mutans, natural honey bought from a local grocery store in Jeddah, Saudi Arabia was compared to artificial honey composed of 40.5% fructose, 33.5% glucose, 7.5% maltose and 1.5% sucrose dissolved in deionized water 1. Different natural and artificial honey concentrations were obtained using serial dilutions with tryptic soy broth (TSB) and at 12.5%, natural honey supported less bacterial growth and biofilm formation than artificial honey with the same amount of sugars, suggesting that sugar content is not the only antibacterial factor 1. Natural honey was able to decrease the maximum velocity of S. mutans growth compared to artificial honey 1. Overall, natural honey demonstrated more inhibition of bacterial growth, viability, and biofilm formation than artificial honey 1.
Microbes Inhibited by Honey
Staphylococcus
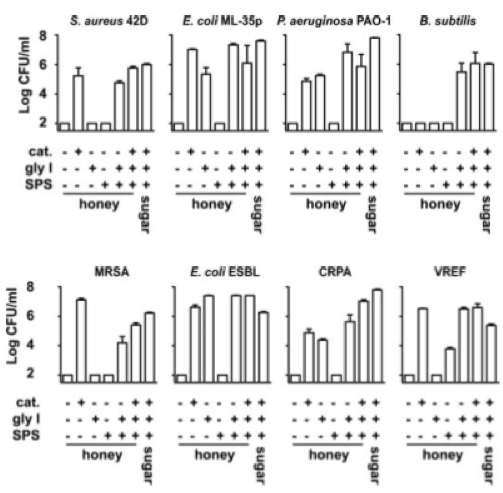
Coagulase-positive Staphylococcus aureus has been shown to be sensitive to both pasture and manuka honeys 4. In this study there was a lack of significant variance in the sensitivity of a large number of clinical isolates collected from a wide range of wounds, which indicates that there is no mechanism of resistance to the antibacterial activity of honey 4. Methicillin-resistant Staphylococcus aureus (MRSA) growth has been shown to be inhibited by Revamil medical honey and manuka honey, as shown in Figure 310.
Streptococcus
Streptococcus mutans growth, viability, and biofilm formation were inhibited by concentrations between 25 and 12.5% of natural honey1. Bacterial growth and biofilm formation were determined using a microplate spectrophotometer on wells inoculated with S. mutans containing varying concentrations of natural and artificial honey1. Biofilms were fixed using formaldehyde solution, followed by crystal violet, and then isopropanol, after that the wells were aspirated and their absorbances were read 1. The number of colony-forming units (CFU) for varying concentrations of honey was determined using an automated colony counter and compared to values from the tryptic soy broth (TSB) control culture to determine the effect of honey on S. mutans viability 1.
Streptococcus pyogenes and Streptococcus faecalis were tested on blood agar and honey-blood agar plates 8 to test for the effectiveness of honey in a diluted, nutrient rich environment. In these tests, Streptococcus pyogenes was inhibited in concentrations of honey below 20%, and all microbes tested were inhibited in solutions with greater than 50% honey content 8.

Other
Unprocessed Revamil source honey effectively killed Bacillus subtilis, Methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase producing Escherichia coli , ciprofloxacin-resistant Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus faecium 3. The activity of honey against E. coli and P. aeruginosa was markedly reduced when either H2O2 or MGO was neutralized 3.
The relationship between the presence of honey and bacterial growth was tested on the following bacteria on nutrient-agar and honey-nutrient agar plates: Vibrio cholerae, enteropathogenic E. coli, Salmonella typhi, Shigella boydii, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Serratia marcescens 8. Staphylococcus aureus and Listeria monocytogenes were tested on blood agar and honey-blood agar plates 8. Finally, chocolate-agar and honey-chocolate agar plates were used to test the growth of Haemophilus influenzae 8. There was good growth of all bacteria on their respective control plates and all intestinal bacterial pathogens tested failed to grow in honey at concentrations of 40% and above 8. Furthermore, the growth of V. cholerae, S. pyogenes, and H. influenzae were inhibited in honey at concentrations as low as 20% and the growth of all bacteria tested was inhibited at honey concentrations of 50% 8.
Langaneza Black Forest honey inhibited the growth of S. pyogenes, E. coli, P. aeruginosa, Candida albicans, Methicillin-sensitive and Methicillin-resistant S. aureus, and Vancomycin-sensitive and Vancomycin-resistant enterococci 7.
Further Reading
Symbionts as Major Modulators of Insect Health Lactic Acid Bacteria and Honeybees Vásquez et al. (2012) found that the crop of bees contains lactic acid bacteria (LAB), the predominant one being Lactobacillus kunkeei (pictured at right) 12. Commercial antibiotics commonly administered to bees were shown to be detrimental for the LAB, and may end up being more of a hindrance than a help for bees. There may very well be a connection between LAB and the antimicrobial effects of honey 12.
Evidence Supporting the Use of Honey as a Wound Dressing Molan (2006) reviewed 17 randomized controlled trials involving a total of 1,965 participants, 5 clinical trials of other forms involving 97 participants treated with honey, and 16 trials on a total of 533 wounds on experimental animals all with findings that demonstrate the effectiveness of honey in assisting wound healing13. This review found that honey has antibacterial activity capable of rapidly clearing infection and protecting wounds from becoming infected, while providing a moist healing environment without the risk of bacterial growth13. This review also reports that honey produces anti-inflammatory effects to reduce edema and exudate and prevent or minimize hypertrophic scarring 13. Honey also stimulates the growth of granulation tissue and epithelial tissue so that healing is hastened13.
Effect of Honey on Wound Healing Time Effects of topical honey on post-operative wound infections due to gram positive and gram negative bacteria following Caesarean sections and hysterectomies were investigated14. Topical application of crude undiluted honey resulted in faster eradication of bacterial infections, reduced period of antibiotic use and hospital stay, accelerated wound healing, reduced occurrence of wound dehiscence and need for re-suturing and minimized scar formation14.
References
11. Bionicgrrrl. "Comb honey from Alois Dallmayr." Flickr (May 16, 2009).
Edited by Celina Hayashi, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.

