Canine Distemper Virus: Wildlife Conservation Implications
By Sam Lisak
Taxonomic Information
Baltimore Classification
Group V: (−) sense single-stranded RNA virus
Higher Order Taxa[1]
Order: Mononegavirales
Family: Paramyxoviridae
Genus: Morbillivirus
Species: Canine Distemper Virus
Negative-Strand RNA Viruses
Negative-sense RNA viruses are a diverse group of pathogens that includes important human pathogens such as parainfluenza viruses, influenza viruses, and respiratory syncytial virus (RSV). This group also contains important viruses for both domestic and wild animals such as Newcastle disease virus (NDV), rinderpest virus (RPV), and, the focus of this report, canine distemper virus (CDV) [2]. Like all negative-sense single-stranded RNA viruses, CDV must be converted to positive sense RNA by RNA-dependent RNA polymerase (RdRp) before it can be translated during replication. However, negative-sense RNA viruses can be further categorized by the segmentation of their genome. As a member of the Paramyxoviridae family, CDV has a nonsegmented genome while other viruses, like influenza (Orthomyxoviridae family), have segmented genomes. The main difference between the two is that nonsegmented genome replication occurs in the host's cytoplasm while segmented genome replication occurs in the host's nucleus [3]. Besides Paramyxoviridae, other families of negative-sense RNA that have nonsegmented genomes are Rhabdoviridae, Filoviridae , and Bornaviridae [2]. Between these families, Rhabdoviridae and Paramyxoviridae possess intermediate genome sizes (10.8-14.9 kb; 15.1-15.9 kb) while Bornaviridae have the smallest genome (8.9 kb) and Filoviridae have the largest genome (19.1 kb) [4]. The Paramyxoviridae family can be further grouped into the subfamily Paramyxovirinae which all have 6-7 transcriptional elements and a closely related amino acid sequence [5]. Among this subfamily, the genus Morbillivirus can be distinguished by a lack of neuraminidase activity: the cleavage of sialic groups from glycoproteins [5]. Neuraminidase activity is important for many negative-sense RNA including influenza viruses where it promotes the release of the virus from infected cells and its spread throughout the respiratory tract [6].
Description and Significance

Canine distemper virus (CDV) is a viral pathogen that is commonly known for its ability to infect domestic dogs, though it affects many other species as well. CDV is highly contagious and often fatal, spreading through aerosol droplets and bodily fluids. Generally, the virus initially replicates in the respiratory tract where it enters the blood stream and spreads to lymphoid, nervous, and epithelial tissues [1]. Although much research on this virus concentrates on its relationship with domestic dogs, CDV affects mammalian families such as Canidae (domestic and wild canids), Ursidae (bears), Pinnipeds (seals, sea lions, etc.), and Felidae (wild cats), to name a few [8]. In fact, every family in the mammalian order Carnivora is susceptible to CDV as well as various families belonging to other mammalian orders (i.e. Rodentia, Primates, Artiodactyla, and Proboscidea) [9]. Due to the wide range of potential hosts and the easy transmission pathway from domestic dogs to wildlife, as well as transmission between wildlife species, CDV poses a significant threat to the conservation efforts of many threatened mammals. Many species belonging to the Carnivora order are inherently vulnerable to extinction in the human-dominated world due to ecological and behavioral traits such as requiring large, connected tracts of habitat and preying on livestock. As such, the additional threat of CDV has important conservation implications. This report attempts to summarize current research on CDV as well as provide insight on how the virus affects wildlife, specifically, the conservation of threatened species.
Virion and Genome Structure
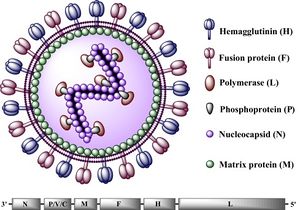
All Morbilliviruses, including CDV, are virions enveloped by a lipid bilayer containing a single-stranded, negative-sense RNA genome [11]. CDV virus particles are typically between 150-300 nm in diameter [10].Their genomes code for six structural proteins in the following order: N (nucleocapsid), P(phosphoprotein), M (matrix protein), F (fusion protein),
H (hemagglutinin protein), and L (large protein) (See Figure 2). They also code for V and C, two non-structural proteins [10]. The P and L proteins are coupled and attached to the N protein, which surrounds the genome to form the nucleocapsid. The P protein serves as the co-factor for the viral RNA-dependent RNA polymerase (L protein) [12]. The two glycoproteins, H and F, are spikes embedded in the lipid bilayer that mediate fusion with other cells. The envelope-associated M protein is evenly arranged underneath the lipid bilayer and regulates the two glycoproteins in addition to linking the nucleoplasmid with these proteins [12].The CDV genome consists of 15,616 nucleotides and parallels the genomes of other Morbilliviruses, particularly that of its closest relative, the measles virus (MV) [13]. This genome begins with the putative regulatory 3' leader (55 nucleotides long) that directly precedes the N gene, which is then followed, sequentially, by the P, M, F, H, and L genes. Between the L gene and the end of the CDV genome (the 5' terminus), lies the putative 5' leader region (38 nucleotides long) [14]. The putative sequences direct the transcription and replication of the genome. Intergenic sequences, located at the 3' and 5' ends of every structural gene segment, control transcription termination and reinitiation [15].
Much of the recent research on the CDV genome has focused on factors that lead to DNA mutation and the evolution of the virus. One study, in particular, made a significant breakthrough regarding the role of homologous recombination in CDV evolution. The researchers conducted phylogenetic and recombinational analyses on 25 full-length CDV genomes and found that mutation and recombination occurred in the coding regions for all six of the structural proteins identified earlier in this report (P, N, L, M, F, and H) [12]. As such, homologous recombination appears to be a key driver of genetic diversity and evolution in CDV.
Viral Ecology and Pathology
While the precise pathology of CDV depends on the infected species, in all species the virus is primarily transmitted through aerosols containing the virus but can also spread through bodily secretions and excretions [17]. Though highly contagious, CDV is typically short-lived in the environment, however low temperatures can prolong the virus' survival to up to 14 days [17]. In the environment, the virus is either transmitted through direct contact (i.e. respiratory inhalations) or indirect contact (feces, urine, etc.) [18]. These potential mechanisms make transmission between unvaccinated domestic dogs almost unavoidable. Domestic dogs are the most common reservoir for spreading CDV to wildlife, however many other reservoirs species exist such as raccoons and coyotes [18] [17]. The virus is even known to spread to wildlife through the predation of reservoir species by wildlife. For example, CDV spread to tigers after they consumed unvaccinated domestic dogs [19].
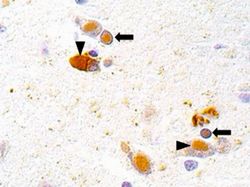
CDV typically enters through the respiratory tract and, within the first 24 hours of infection, replicates in macrophages, spreading to the tonsils and bronchial lymph nodes through local lymphatics [20]. Replication of CDV in the tonsils and bronchial lymph nodes occurs 2-4 days after the initial infection. Around 4-6 days after the initial infection, the virus spreads throughout the lymphoid system [20]. The virus spreads to the central nervous system (CNS) and epithelial tissue about 8-10 days after infection [17]. Recent research tested for the presence of CDV in a spotted hyena (Crocuta crocuta) from Seregeti National Park using immunohistochemistry. Viral antigen was found in the nuclei and cytoplasm of neuronal and glial cells, demonstrating the spread of the virus to the CNS (See Figure 3) [16]. The development of the canine distemper disease depends on the ability of the host immune response to clear the virus from the body's tissues. Individuals possessing cell-mediated toxicity and sufficient levels of antibody titers will usually be able to eliminate the virus from their tissues without showing signs of the disease. However, in individuals with an insufficient immune response, the virus will spread through their tissues, causing clinical signs of the disease to surface [17].
Signs and Symptoms of Canine Distemper
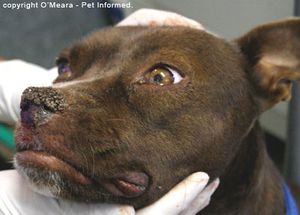
The exact symptoms of CDV vary by the infected species, strain virulence, environmental conditions, host age, and host immune status. However, in all species, the virus targets the respiratory, CNS, integumentary, and gastrointestinal systems [17]. Generalized symptoms of affected animals across all carnivore families include diarrhea, neurological issues, impaired immune function, and dyspnea [16]. Symptoms common among Canidae include pneumonia, vomiting, anorexia, dehydration, fever, ocular discharge, and the thickening of feet pads [20] [22]. However, infections can be difficult to detect among Felids as CDV often causes clinically silent infections and fatal disease [16]. For most carnivore families, infection of the CNS can also lead to neurological symptoms such as muscle twitching, seizures, and occasionally paralysis which is usually followed by death (See Figure 4) [23] [24]. CDV survival rates depend, in large part on the host species. For example, the fatality rate of infected domestic ferrets is close to 100% while 50-70% of infected domestic dogs don't even show symptoms [17].
Given the wide array of symptoms associated with CDV infection, it can be difficult to diagnose. Many diseases could potentially cause many of these same symptoms, particularly diseases that also infect the respiratory, CNS, integumentary, and gastrointestinal systems. For definitive diagnosis of CDV, more in depth methods are required. Unfortunately, the diagnosis of CDV infection in previously vaccinated or infected individuals through serological methods can be difficult due to the potentially high antibody titers of the virus [25]. Additionally, fluorescent antibody (FA) staining in epithelial cells can produce a definitive diagnosis of CDV [25]. However, this procedure often takes several days to weeks which not a practical timescale in terms of catching and treating the disease early. Due to CDV's high mortality rates and potential to rapidly spread, a quick, sensitive method for CDV detection is required to successfully contain the virus. The most successful method for the rapid detection of CDV infection is reverse-transcriptase-polymerase chain reaction (RT-PCR). Currently the most sensitive and effective technique for CDV detection, RT-PCR can reverse transcribe target RNA into DNA from a variety of sources such as the potential host's blood, cerebrospinal fluid (CSF), and urine [26]. However, urine is easy to collect and is more useful in antemortem diagnosis of CDV infection. Researchers conducted a prospective study on the effectiveness of RT-PCR to diagnose canine distemper virus from urine samples. They found that urine is more sensitive than blood samples and at least as sensitive as CSF in the detection of CDV, further supporting the use of urine as the primary source for RT-PCR [26].
Prevention and Treatment of Canine Distemper
The prevention of CDV, while simple in theory, is almost impossible to successfully implement. Effective vaccines exist and are the only realistic solution for controlling canine distemper in domestic dogs [28]. While CDV vaccines have greatly decreased the prevalence of canine distemper in domestic dogs, the disease still spreads among unvaccinated populations including animal shelters, pet stores, and feral dogs. Additionally, many domestic dogs are suceptible to CDV if their owner forgets or declines to vaccinate their puppies with the required booster shots that are needed until the puppy reaches 16 weeks [29]. Prevention of CDV in captive wild animals can be achieved through careful vaccination, however, these vaccines must be proven to be safe for the species in question as the vaccines can occasionally have adverse effects [28]. In fact, modified-live vaccines have been known to induce CDV in nondomestic canids. Recombinant vectored vaccines are effective in many nondomestic canids, however, these vaccines must be carefully studied and implemented for each species they are used on [30]. The widespread vaccination of wild animals is obviously unfeasible, so effective conservation and wildlife management strategies must be implemented to prevent the transfer of CDV to wildlife. However, the direct vaccination of wildlife can potentially prevent the contraction of CDV for small, concentrated populations [31].
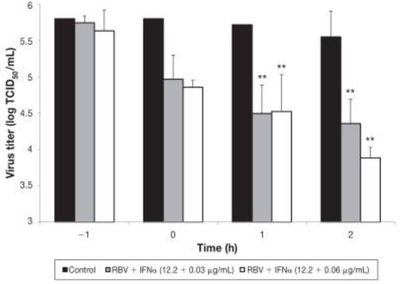
Despite the widespread prevalence of CDV vaccinations, there are currently no antiviral drugs or specific treatments for canine distemper [20]. Available treatments are symptomatic, aimed at controlling neurological dysfunction, maintaining fluid balance, and defending against secondary bacterial invasions [32]. Potential treatments include electrolyte solutions, broad-spectrum antibiotics, parenteral nutrition, antipyretics, analgesics, and anticonvulsants. However, once acute neurological symptoms of CDV appear, recovery is extremely rare [32]. Even after gastrointestinal and respiratory symptoms fade, neurological manifestations often persist. Recent research has focused on developing effective antiviral medication to treat CDV. One study aimed to test the efficacy of the antiviral action of the drugs ribavarin (RBV) and interferon-alpha (IFNα) against CDV. The researchers inoculated Vero cells with CDV and treated them with both of these drugs, as well as combinations of the two. They found that both drugs were able to inhibit viral replication in vitro and that combinations of the two displayed the highest antiviral activity (See Figure 5) [27]. These results are promising for the development of an antiviral medication, though further research on the antiviral efficacy of these drugs is needed to assess their clinical applicability.
Effect on Wildlife Conservation
The ability of CDV to infect all terrestrial carnivore families (Mustelidae, Ursidae, Viverridae, Procyonidae, Felidae, Hyaenidae, and Canidae), in addition to various non-carnivores, allows it to threaten the conservation efforts and long-term survival of many species [17] [33]. Large predators are frequently in danger of extinction due to their reliance on large tracts of habitat, vulnerability to poaching, and frequent conflicts with humans. Exposure to CDV only exacerbates the pressure on these struggling populations of carnivores. Part of what makes CDV so dangerous to wildlife populations is its ability to spread between many species [34]. Domestic dogs are the most common reservoir for the transmission of CDV to wildlife populations. However, infected individuals of the same species in addition to other wild carnivores can also spread the virus. As the human population continues to expand, the threat of CDV to wildlife populations only grows [30]. One example of the growing threat of CDV comes from a longitudinal study on the prevalence of canine distemper in domestic dogs that border the Masai Mara National Reserve, Kenya. This study found that antibody prevalence to CDV rose from just 4% in 1989 to 75% in 1991 [35]. Following this rise, mortality in domestic dogs rose and the sightings of wild canids in the reserve such as jackals and bat-eared foxes (Otocyon megalotis) declined. As the prevalence of CDV in reservoir species continues to grow, so will the danger posed towards wildlife populations.

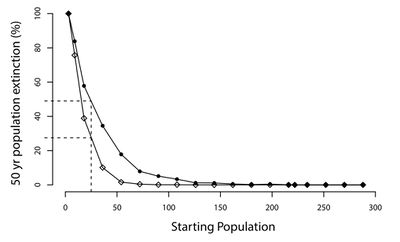
CDV has repeatedly demonstrated the ability to drive already threatened species further towards extinction. A study on a population of Amur tigers (Panthera tigris altaica) in the Sikhote-Alin Biosphere Zapovednik (SABZ) assessed the impact of CDV on this population in addition to modelling how CDV could influence the potential extinction rates of tiger populations of various sizes. Their model found that CDV prevalance could increase the 50-year extinction probability of the tiger population in SABZ by 6.3% to 55.8% [19] (See Figure 6). The most important factors determining the model's outcome was the prevalence of CDV in reservoirs (domestic dogs and other wild carnivores) and its rate of effective contact with tigers [19]. Many endangered species only persist in small populations (relative to former levels), making them particularly susceptible to additional threats like CDV. Given the ability for CDV to jump from domestic dogs to wildlife populations, many studies have focused on monitoring populations of domestic dogs that could potentially come into contact with target species. A recent study examined the ability for CDV transmission from domestic dogs to threaten giant panda (Ailuropoda melanoleuca) populations. Their study population of pandas was found to be extremely susceptible to CDV due to a lack of an antibody for the virus in addition to living in close proximity to unvaccinated domestic dogs that have been exposed to CDV [33]. While CDV has not yet infected this population of pandas, other species have experienced large scale declines in population density directly due to CDV outbreaks. In 1994, one third of all lions (Panthera leo) in the Serengeti died from CDV, an unprecedented occurrence as prior to the outbreak, CDV had never been detected in wild lions [34]. The presence of CDV in lions was a bit puzzling given domestic dogs and lions rarely come into contact with each other. However, a study found that the virus likely spread from domestic dogs to hyenas (who often commute long distances to agricultural areas) outside the national park. The hyenas then reentered the park and spread the virus to lions [34]. This potential transmission route exemplifies why CDV can be so dangerous for endangered species: so many species can carry the virus, allowing it to spread very easily. Once the virus spreads throughout multiple species in any given ecosystem, containing it is very difficult. Another study aimed determine whether the same strain of the virus is being transmitted between Serengeti carnivores including lions, spotted hyena (Crocuta crocuta), bat-eared fox (Otocyon megalotis), and domestic dogs (Canis familiaris). After comparing the CDV H and P gene sequences isolated from all of these species, they found all of these carnivores exhibited a genetically indistinguishable strain of CDV [37]. As such, it is clear that CDV is, indeed, spreading throughout the Serengeti ecosystem via transmission between various species.
As more and more species are pushed to the brink of extinction, conservationists are increasingly relying on ex situ conservation strategies such as captive breeding programs as last-ditch efforts to save species. Captive breeding programs can be used to establish new populations of endangered species, bolster existing ones, and preserve species' genetic diversity [38]. However, CDV not only impedes the in situ conservation efforts described above, but has devastated various captive breeding programs as well. The African wild dog (Lycaon pictus) is a critically endangered species of Canidae native to sub-Saharan Africa. In 2000, a captive breeding program of African wild dogs in Tanzania experienced an outbreak of CDV, killing 49 of 52 animals in only two months [39]. The importance of a successful breeding program is demonstrated by the rescue of the black-footed ferret (Mustela nigripes) from the brink of extinction. In 1964, scientists were ready to declare this species extinct when a small population was discovered. This population continued to decline and, in 1971, scientists started a captive breeding program after capturing individual from this population [40]. However, the majority of these captive individuals were infected with CDV and died out, as did the wild population. Conservationists received a second chance to save this species in 1981 when another population was discovered in Wyoming. Unfortunately, CDV wiped out most of this population and, in 1986, the last 18 individuals were captured to start a captive breeding program [41]. Scientists were able to prevent the outbreak of CDV in this population with terrific results. While this species is still endangered, it represents one of the greatest conservation success stories. As of 2017, there are ~300 individuals in captive breeding programs and hundreds of individuals in the wild; 4,100 ferrets have been reintroduced to the wild since 1991 [42]. Though frequent reliance on captive breeding programs will result in failures to rescue threatened species, these programs can potentially reinforce and rebuild populations of critically endangered species in the short term. Clearly, it is essential to prevent the spread of CDV to captive breeding programs if they are to successfully aid conservation efforts.
Potential Conservation Solutions
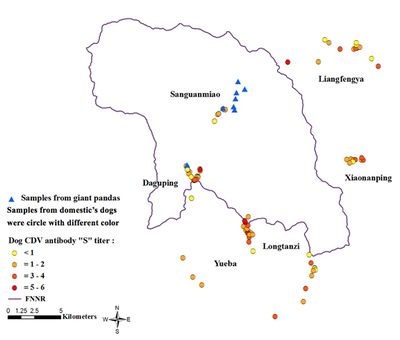
Two main strategies exist for the limitation of CDV outbreaks among endangered species: the vaccination of reservoirs like domestic dogs or the direct vaccination of wildlife. The creation of CDV vaccination buffer zones around vital populations of endangered species has the potential to reduce the frequency of CDV outbreaks. In 1996, after detrimental CDV outbreaks among populations of lions (Panthera leo) and African wild dogs (Lycaon pictus) in the Serengeti ecosystem, a project aimed to vaccinate domestic dogs around the national park was implemented [43]. The overall success of this project is still unclear as there has been at least one outbreak of CDV (of much lower severity) since the start of the program, but some results have been encouraging as the population has recovered to levels higher than before the outbreak. The potential reason for the persistance of CDV in this population is that it has spread to other wildlife in the ecosystem such as spotted hyena (Crocuta crocuta) and bat-eared fox (Otocyon megalotis) [43]. It will be increasingly important to establish effective buffer zones as human expansion continues to threatened endangered wildlife. Assessing the proximity of key populations of endangered species to potential reservoirs of CDV is of great importance. For example, a recent study evaluated the exposure to CDV in 8 giant pandas and 125 unvaccinated domestic dogs located in and adjacent to the Foping National Nature Reserve (FNNR), China [33]. Using RT-PCR assays, researchers found that while the pandas were naive to CDV, many dogs demonstrated previous exposure to the disease with significant antibody titers. When mapping the locations of the pandas and the domestic dogs, it is clear that these pandas are in close proximity to these potentially CDV carrying dogs and are in danger of contracting the disease (Figure 8) [33]. For this population of pandas, as well as other imperiled species and populations, measures can be taken to either vaccinate potential reservoirs, prevent their contact with the target wildlife species, or directly vaccinate the target wildlife species.
The direct vaccination of wildlife has proven effective both as a preventative measure and as a measure of last resort to protect small populations of endangered species severely threatened by CDV. A study examining the preventative vaccination of southern sea otters (Enhydra lutris nereis) found it both safe and effective [44]. Direct vaccination of wildlife has also proven effective when applied to small populations where CDV is a critical factor influencing mortality rates. In 1999, the Santa Catalina Island Fox (Urocyon littoralis catalinae) population experienced a drastic decline due to CDV. The Catalina Island Fox Recovery Plan used direct vaccination, captive breeding, reintroduction, and population monitoring to grow the island's fox population from as low as 100 to ~2,000 (as of 2017) [31]. Additionally, the vaccination of captive endangered species is crucial for the long-term success of captive breeding programs. However, for nondomestic canid and many other wildlife species, modified-live vaccines are dangerous and can even induce CDV [30]. As such, recombinant vectored vaccines can provide an alternative for these species. Recombinant vectored vaccines are currently used safely on captive tigers [30]. However, just because a specific CDV vaccine is effective for one species does not mean it will be effective for another. The crash of the African wild dog (Lycaon pictus) population in a captive breeding program (detailed earlier in this report) was partly a result of the use of an effective CDV vaccine for harbor seals (Phoca vitulina) that failed for the wild dogs [39]. As such, extensive research on the efficacy of specific vaccines for each species of interest is necessary. The development and administering of safe, effective vaccines for both wild and captive animals will be essential to the conservation efforts of many endangered species.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2018, Kenyon College.
- ↑ 1.0 1.1 Pratakpiriya, W., Teh, A.P.P., Radtanakatikanon, A., Pirarat, N., Lian, N.T., Takeda, M., Techangamsuwan, S., & Yamaguchi, R. (2017) Expression of canine distemper virus receptor nectin-4 in the central nervous system of dogs. Scientific Reports, 7(349), 1-9. doi: 10.1038/s41598-017-00375-6
- ↑ 2.0 2.1 Neumann, G., Whitt, M. A., & Kawaoka, Y. (2002). A decade after the generation of a negative-sense RNA virus from cloned cDNA – what have we learned? Journal of General Virology, 83, 2635-2662.
- ↑ Conzelman, K. (1998). Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annual Review Of Genetics, 32, 123-162.
- ↑ Wang, L., Yu, M., Hansson, E., Pritchard, I., Shiell, B., Michalski, W.P., & Eaton, B. T. (2000). The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. Journal of Virology, 74(21), 9972-9979.
- ↑ 5.0 5.1 Paramyxoviridae. International Committee on Taxonomy of Viruses (ICTV), ICTV, talk.ictvonline.org/ictv-reports/ictv_9th_report/negative-sense-rna-viruses-2011/w/negrna_viruses/199/paramyxoviridae.
- ↑ Gubareva, L. V., Kaiser, L., & Hayden, F. G. (2000). Influenza virus neuraminidase inhibitors. Lancet, 355, 827-835.
- ↑ Letxter, R. (2018). Virus Terrible for Dogs Shows up in Critically Endangered Leopard. The Washington Post, WP Company. www.washingtonpost.com/national/health-science/virus-terrible-for-dogs-shows-up-in-critically-endangered-leopard/2018/01/19/3e02a452-fc65-11e7-ad8c-ecbb62019393_story.html?utm_term=.09fc56e9ba47.
- ↑ Yuan, C., Liu, W., Wang, Y., Hou, J., Zhang, L., & Wang, G. (2017). Homologous recombination is a force in the evolution of canine distemper virus. PLoS ONE, 12(4), 1-16. doi: 10.1371/journal.pone.0175416
- ↑ Martinez-Gutierrez, M., & Ruiz-Saenz, J. (2016). Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Veterinary Research, 12, 78. http://doi.org/10.1186/s12917-016-0702-z
- ↑ 10.0 10.1 10.2 Pardo, I. D. R., Johnson, G. C., & Kleiboeker, S. B. (2005). Phylogenetic characterization of canine distemper viruses detected in naturally infected dogs in North America. Journal of Clinical Microbiology, 43(10), 5009–5017. http://doi.org/10.1128/JCM.43.10.5009-5017.2005
- ↑ Sato, H., Yoneda, M., Honda, T., & Kai, C. (2012). Morbillivirus receptors and tropism: multiple pathways for infection. Frontiers in Microbiology, 3(75), 1-9.
- ↑ 12.0 12.1 12.2 Yuan, C., Liu, W., Wang, Y., Hou, J., Zhang, L., & Wang, G. (2017). Homologous recombination is a force in the evolution of canine distemper virus. PLoS ONE, 12(4), e0175416. http://doi.org/10.1371/journal.pone.0175416
- ↑ Mingwei, T., Yi, L., & Cheng, S. (2015). Complete genome characterization of a canine distemper virus isolated from Chines racoon dog in China. Asian Journal of Animal and Veterinary Advances, 10(1), 43-47.
- ↑ Sidhu, M.S., Menonna, J.P., Cook, S.D., Dowling, P.C., & Udem, S.A. (1993). Canine distemper virus L Gene: sequence and comparison with related viruses. Virology, 193(1), 50-65.
- ↑ Sidhu, M.S., Husar, W., Cook, S.D., Dowling, P.C., & Udem, S.A. (1993). Canine distemper terminal and intergenic non-protein coding nucleotide sequences: completion of the entire CDV genome sequence. Virology, 193(1), 66-72.
- ↑ 16.0 16.1 16.2 16.3 Beineke, A., Baumgartner, W., & Wohlsein, P. (2015). Cross-species transmission of canine distemper virus—an update. One Health, 1, 49-59.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Deem, S.L., Spelman, L.H., Yates, R.A., & Montali, R.J. (2000). Canine distemper in terrestrial carnivores: a reveiw. Jounral of Zoo and Wildlife Medicine, 31(4), 441-451.
- ↑ 18.0 18.1 Bronson, E., Emmons, L.H., Murray, S., Dubovi, E.J., & Deem, S.L. (2008). Serosurvey of pathogens in domestic dogs on the border of Noel Kempff Mercado National Park, Bolivia. Journal of Zoo and Wildlife Medicine, 39(1), 28-36.
- ↑ 19.0 19.1 19.2 19.3 Gilbert, M., Miquelle, D.G., Goodrich, J.M., Reeve, R., Cleaveland, S., Matthews, L., & Joly, D.O. (2014) Estimating the potential impact of canine distemper virus on the Amur tiger population (Panthera tigris altaica) in Russia. PLoS ONE 9(10): e110811. doi:10.1371/journal.pone.0110811
- ↑ 20.0 20.1 20.2 20.3 Carvalho, O.V., Botelho, C.V., Ferreira, C.G.T., Scherer, P.O., Soares-Martins, J.A.P., Almeida, M.R., & Junior, A.S. (2012). Immunopathogenic and neurological mechanisms of canine distemper virus. Advances in Virology, 1-10.
- ↑ Veterinary Advice Online: Canine Distemper Virus. Pet Informed , www.pet-informed-veterinary-advice-online.com/canine-distemper.html.
- ↑ V. Martella, F. Cirone, G. Elia, E. Lorusso, N. Decaro, M. Campolo, C. Desario, M.S. Lucente, A.L. Bellacicco, M. Blixenkrone-Møller, L.E. Carmichael, & C. Buonavoglia. (2006). Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Veterinary Microbiology, 116(4), 301-309. doi: 10.1016/j.vetmic.2006.04.019
- ↑ Fix, A.S., Riordan, D.P., Hill, H.T., Gill, M.A., & Evans, M.B. (1989). Feline panleukopenia virus and subsequent canine distemper virus infection in two snow leopards (Panthera uncia). Journal of Zoo and Wildlife Medicine, 20(3), 273-281.
- ↑ Carpenter, M.A., Appel, M.J.G., Roelke-Parker, M.E., Munson, L., Hofer, H., East, M., & O'Brien, S.J. (1998) Genetic characterization of canine distemper virus in Serengeti carnivores. Veterinary Immunology and Immunopathology, 65(2-4), 259-266.
- ↑ 25.0 25.1 Kim, Y.H., Cho, K.W., Youn, H.Y., Yoo, H.S., & Han, H.R. (2001) Detection of canine distemper virus (CDV) through one step RT-PCR combined with nested PCR. Journal of Veterinary Science, 2, 59-63.
- ↑ 26.0 26.1 Saito, T.B., Alfieri, A.A., Wosiacki, S.R., Negrao, F.J., Morais, H.S.A., & Alfieri, A.F. (2005). Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Research in Veterinary Science, 80, 116-119.
- ↑ 27.0 27.1 Carvalho, O.V., Saraiva, G.L., Ferreira, C.G.T., Felix, D.M., Fietto, J.L.R., Bressan, G.C., Almeida, M.R., & Junior, A.S. (2014). In-vitro antiviral efficacy of ribavirin and interferon-alpha against canine distemper virus. Canadian Journal of Veterinary Research, 78(4), 283-289.
- ↑ 28.0 28.1 Chappuis, G. (1995). Control of canine distemper. Veterinary Microbiology, 44(2-4), 351-358.
- ↑ Day, M.J. (2014) Canine vaccination guidelines. VacciCheck, 551-554.
- ↑ 30.0 30.1 30.2 30.3 Seimon, T.A., Miquelle, D.G., Chang, T.Y., Newton, A.L., Korotkova, I., Ivanchuk, G., Lyubchenko, E., Tupikov, A., Slabe, E., & McAloose D. (2013). Canine distemper virus: an emerging disease in wild endangered Amur tigers (Panthera tigris altaica). mBio 4:e00410-13. https://doi.org/10.1128/mBio.00410-13.
- ↑ 31.0 31.1 Catalina Island Conservancy: Foxes. 2018. Long Beach (CA): Catalina Island Conservancy. http://www.scientificstyleandformat.org/Tools/SSF-Citation-Quick-Guide.html
- ↑ 32.0 32.1 Creevy, K.E. Canine Distemper Overview - Generalized Conditions. Merck Veterinary Manual.
- ↑ 33.0 33.1 33.2 33.3 33.4 Jin, Y., Zhang, X., Ma, Y., Qiao, Y., Liu, X., Zhao, K., Zhang, C., Lin, D., Fu, X., Xu, X., Wang, Y., & Wang, H. (2017). Canine distemper viral infection threatens the giant panda population in China. Oncotarget, 8(69), 113910-113919.
- ↑ 34.0 34.1 34.2 Craft, M.E., Ecology of infectious diseases in Serengeti lions. Biology and Conservation of Wild Felids, 263–282. Oxford, Oxford University Press.
- ↑ Alexander, K. A. & Appel, M. J. G. (1994). African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. Journal of Wildlife Diseases, 30(4), 481-485.
- ↑ Releasing Black-footed ferrets into the wild. (2014). Smithsonian's National Zoo & Conservation Biology Institute. Washington, DC.
- ↑ Carpenter, M. A., Appel, M. J. G., Roelke-Parker, M. E., Munson, L., Hofer, H., East, M., & O'Brien. (1998). Genetic characterization of canine distemper virus in Serengeti carnivores. Veterinary Immunology and Immunopathology, 65(2-4), 259-266.
- ↑ Fraser, D.J. (2008). How well can captive breeding programs conserve biodiversity? A review of salmonids. Evolutionary Applications, 1(4), 535–586. http://doi.org/10.1111/j.1752-4571.2008.00036.x
- ↑ 39.0 39.1 Van de Bildt, M.W.G., Kuiken, T., Visee, A.M., Lema, S., Fitzjohn, T.R., & Osterhaus, A.D.M.E. (2002). Distemper outbreak and its effect on African wild dog conservation. Emerging Infectious Diseases, 8(2), 212–213. http://doi.org/10.3201/eid0802.010314
- ↑ US Fish and Wildlife Service. (1988) Black-footed ferret recovery plan. US Fish and Wildlife Service, Denver, Colorado.
- ↑ Roelle, J.E., Miller, B.J., Godbey, J.L., & Biggins, D.E. (2004) Recovery of the Black-footed ferret: progress and continuing challanges. Scientific Investigations Report 2005-5293.]
- ↑ Howard, J.G., Lynch, C., Santymire, R.M., Marinari, P.E., & Wildt, D.E. (2015). Recovery of gene diversity using long‐term cryopreserved spermatozoa and artificial insemination in the endangered black‐footed ferret. Animal Conservation, 19(2), 118-119.
- ↑ 43.0 43.1 Chauvenet, A.L.M., Durant, S.M., Hilborn, R., & Pettorelli, N. (2011). Unintended consequences of conservation actions: managing disease in complex ecosystems. PLoS ONE 6(12): e28671.
- ↑ Jessup, D.A., Murray, M.J., Casper, D.R., Brownstein, D., & Kreuder-Johnson, C. (2009). Canine distemper vaccination is a safe and useful preventative procedure for southern sea otters (Enhydra lutra nereis). Journal of Zoo and Wildlife Medicine, 40(4), 705-710.
