Equine cecum
The Equine Digestive Tract
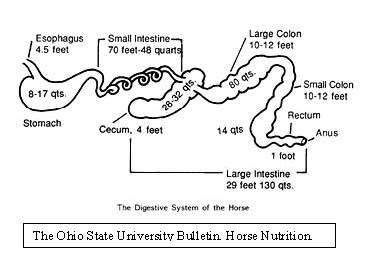
The microbial niche created by the equine cecum is part of a complex and delicate ecosystem. The cecum is commonly referred to as the fermentation vat of the equine digestive system. The fermentation is carried out by microbes that inhabit this environment; mainly by bacteria, but also by some protozoans and some vegetative growth of phycomycete fungi. (Mackie, 1997) These microbes are a vital part of the digestive system of horses, with the cecum accounting for approximately 15% of the digestive tract. (Wright) The environmental conditions of the cecum directly effect the type of microbes that can survive there. The cecum is located between the stomach and the colon and is classified as part of the large intestine. The pH of the cecum is relatively neutral; the stomach acidic and the colon alkaline. pH fluctuations in either direction can result in changes in the populations of microbes in the cecum. (Harman) The cecum is four feet long and has a capacity of about 26 to 32 liters. Both the entrance from the stomach and the exit to the colon are located at the top and rest of the cecum extends down from there. This pocket like structure ties in with the relatively long time, approximately 7 hours, that contents take to pass through the cecum and the relatively low motility in this part of the GI tract, which helps microbes stay in place. (Tyler)
Primary Microbial Residents of the Equine Cecum
The natural microflora of the equine cecum includes both gram positive and gram negative bacteria as well as several species of protozoa. The cecum contains several lactic acid producing species in the genus Streptococcus, including Streptococcus bovis http://microbewiki.kenyon.edu/index.php/Streptococcus and Streptococcus equinus. S. bovis is actually the most prolific species in the cecum. Other lactic acid producers located in the cecum are members of the genus Lactobacillus http://microbewiki.kenyon.edu/index.php/Lactobacillus. Several species of gram negative Bacteroides http://microbewiki.kenyon.edu/index.php/Bacteroides are also found in the cecum. (Mackie)
Bacterial Metabolism
The microflora located in the cecum provide several necessary functions for the horse. First and foremost, several of the bacterial species are involved in fermenting complex polysaccharides, changing them into a form that is digestible by the horse. Cellulolysis in the cecum is not currently a well understood process; however, experiments by Julliand et al. have discovered the major bacteria responsible for cellulolysis. Using oligonucleotide probes, they discovered three major species of cellulolytic bacteria. The predominant species found was Ruminococcus flavefaciens http://microbewiki.kenyon.edu/index.php/Ruminococcus. Two other species of cellulolytic bacteria were also found to be present in low levels: Fibrobacter succinogens and Ruminococcus albus. While all three of these species of bacteria are also found in the bovine rumen, Julliand et al. discovered that the equine strains differed from bovine strains in the way they utilized carbohydrates and in the end products they produced. (Julliand)
The main products of fermentation of complex polysaccharides are volatile fatty acids (VFAs). The VFAs diffuse into the blood and are the main source of energy for the horse. The main VFAs produced are acetate, propionate and butyrate with isobutyrate, isovalerate and valerate produced in smaller amounts. (Mackie, 1988) These VFAs also serve to lower the pH of the cecum helping to prevent the growth of unwanted microflora such as Salmonella http://microbewiki.kenyon.edu/index.php/Salmonella. High concentrations of VFAs also inhibit the growth of Escherichia coli http://microbewiki.kenyon.edu/index.php/Escherichia_coli, which explains why they are not found in the cecum. (Kern, 1973)
While the cecum contains several species of bacteria capable of producing lactic acid, the concentration of lactic acid is normally kept relatively low. The lactic acid fermenters use simple sugars to create the lactic acid; however, most simple sugars are digested enzymatically before they reach the cecum.
Another major function of the bacteria in the cecum is to break down amino acids. By the time nutrients reach the cecum, approximately fifty percent of ingested protein has been digested enzymatically. The remaining protein is digested by proteolytic bacteria in the hindgut. This serves to provide valuable nutrients for the horse. Some bacteria in the cecum are capable of decarboxylating amino acids, which provide a source of nitrogen for the horse. (Bailey) Certain bacteria are also able to create vitamins, such as vitamin B, for the horse’s use.
Host-Bacteria Symbiosis - A Dynamic Microbial Population
The bacteria located in the cecum are reliant on their host for protection as well as for nutrition. The horse’s diet provides the microbes in the cecum with an energy resource unusable by the horse. In turn, the end products of the microbes’ metabolism provide the horse with an energy resource. When a horse’s diet changes, so does the composition of the microbes in the cecum. In a beautiful example of symbiosis, the microflora adapt to the new nutrients provided for them by the food the horse eats. Different species of bacteria will proliferate and others will die off depending on their ability to metabolize the new nutrients.
Systemic Effects Resulting from Cecal Microflora Imbalance
Equines rely on many microbes in the cecum, as discussed above, to aid in digestion and to make nutrients available that would otherwise be unavailable through enzymatic digestion alone. It has been observed that disruptions to the delicate balance of cecal microflora can have systemic effects on equines, including changes in fecal and blood pH levels, diarrhea, weight loss and lameness due to laminitis.
One common source of microbial imbalance occurs when a horse ingests a large amount of cereal grains or grass that is too rich in carbohydrates or starch. Such a carbohydrate overload is common in pasture-fed horses during the spring and summer when grass grows very rapidly. It is also common when horses are fed large amounts of cereal grains. When a sudden increase in the amount of fermentable material in the cecum occurs, large blooms of gram positive bacteria such as Lactobacillus and Streptococcus bovis may occur, resulting in an increase in lactic acid production and an overall change in bacterial populations as pH levels decrease. (Rowe)
In an experiment conducted by Pollitt et al., eight horses were randomly allocated to three treatment groups and one control group. The treatment group horses were dosed with 7.2, 10 and 12.5 g/kg treatments of oligofructose dissolved in water. All of the treated horses developed diarrhea, pyrexia, elevated heart rate, loss of bicarbonate, and laminitis. And, because mammals produce only the L-isomer of lactate and bacteria produce both the D-lactate and L-lactate isomers, researchers were able to correlate these symptoms with increased bacterial populations, including S. bovis and S. equinas, as measured by D-lactate concentration in the blood. These gram positive bacteria are known to produce lactic acid and therefore to decrease pH levels in the cecum and increase lactic acid in the blood. The researchers also found that D-lactate disappeared from blood samples at 40 hours post treatment, indicating a sharp decline in D-lactate producing organisms after an initial, very rapid increase immediately following dosing of the oligofructose.
In a similar experiment conducted by Rowe et al., twelve horses were divided into three treatment groups. All treatment groups received pelleted grain diets, but two of the groups also received a feed additive containing the antibiotic Virginiamycin, which is known to inhibit growth of gram positive bacteria such as S. bovis and Lactobacillus. D-lactate blood concentration, fecal pH, weight and lameness were evaluated in all horses. The researchers found that D-lactate levels peaked on day 3 and day 8 in the horses that did not receive Virginiamycin and that D-lactate levels in the horses receiving the antibiotic did not change significantly. In addition, fecal pH decreased sharply in the untreated horses (P<0.05) and lameness scores increased (P<0.001) in the untreated horses versus those receiving the antibiotic. The results of this study appear to show a clear link between the presence of gram positive, lactic acid producing bacteria in the hindgut and the appearance of potentially serious systemic symptoms in equines.
In addition to decreasing cecal pH levels, it has been found that lactic acid producing gram positive bacteria also produce decarboxylase enzymes that convert free amino acids into monoamines. An imbalance of monoamines may pose a risk to equines because they can mimic naturally occurring amines such as serotonin, epinephrine and dopamine in the blood, resulting in excessive vasoconstriction, especially in the digits. (Elliott et al.) In order to provide evidence to support this claim, Elliott et al. created an in vitro model of the equine cecum and cultured cecal contents anaerobically while simulating a carbohydrate overload through the addition of inulin. The researchers’ findings confirmed a decrease in pH level and an increase in phenylethylamine and isoamylamine concentrations. In addition, S. bovis and Lactobacillus were isolated from the simulated cecal environment. The addition of Virginiamycin to the simulated cecal contents was effective in inhibiting both the decrease in pH levels and the increase in amine production, indicating the bacteria were responsible for both conditions.
Discussion
As discussed, the equine cecum contains a delicate balance of many different gram negative and gram positive microcrobes. Under normal conditions, these bacteria help provide nutrients for the horse and the horse provides protection and nutrients for the bacteria living within it. These symbiotic relationships have developed over time to provide the greatest benefit for all organisms involved under natural conditions for the horse, which is physically adapted to graze and to take in forage that is not too rich in nutrients. Domestication, and the resulting carbohydrate overload from food sources such as rich pasture and grain, has led to imbalances in the cecal microflora and negative systemic effects for the horse. Developing a better understanding of the equine cecal microflora will help humans avoid such negative, and potentially life threatening, effects in our horses
References
“Explaining Laminitis and Founder Part Two: What Causes Laminitis?” The Cyberhorse Guide To Horse Health. 2003. CyberHorse. 2003 <http://www.cyberhorse.net.au/cgi-bin/tve/displaynewsitem.pl?20040325laminitispt2.txt>.
Bailey, S. R., M. L. Baillon, A. N. Rycroft, P. A. Harris, and J. Elliot. “Identification of Equine Cecal Bacteria Producing Amines in an In Vitro Model of Carbohydrate Overload.” Applied and Environmental Microbiology. Apr. 2003. p2087-2093.
Elliott, Jonathan and Simon R. Bailey. “Gastrointestinal Factors Are potential Triggers for the Development of Acute Equine Laminitis”. The Journal of Nutrition. July 2006. Volume 136. p. 2103S-2107S.
Harman, Joyce. “Holistic Equine Nutrition”. Harmony Equine Clinic. http://www.harmanyequine.com/holistic_equine_nutrition.stm 2008.
Howard, Tyler. “The Equine Digestive System”. www.anslab.iastate.edu/Class/AnS320/19%20Equine%20Nutrition.ppt.
Hussein, H.S. and L.A. Vogedes. “Review: Forage Nutritional Value for Equine as Affected by Forage Species and Cereal Grain Supplementation”. Professional Animal Scientist. October 2003.
Julliand Veronique, Albane de Vaux, Liliane Millet, and Gerard Fonty. “Identification of Ruminococcus flavefaciens as the Predominant Cellulolytic Bacterial Species of the Equine Cecum.” Applied and Environmental Microbiology. Aug. 1999. p3738-3741.
Kern, D.L., L. L. Slyter, E.C. Leffel, J. M. Weaver and R. R. Oltjen. “Ponies vs. Steers: Microbial and Chemical Characteristics of Intestinal Ingesta.” Journal of Animal Science. 1974. 36:559-564.
Kern, D.L., L. L. Slyter, J. M. Weaver, E. C. Leffel, G. Samuelson. “Pony cecum vs. Steer Rumen: The Effect of Oats and Hay on the Microbial Ecosystem.” Journal of Animal Science.1973. 37:46-469.
Kline, Robert, Porr, Shea and Cardina, John. The Ohio State University Bulletin. Horse Nutrition. Bulletin 762-00. http://ohioline.osu.edu/b762/b762_5.html.
Mackie, Roderick I., and Clive A. Wilkins. “Enumeration of Anaerobic Bacterial Microflora of Equine Gastrointestinal Tract.” Applied and Environmental Microbiology. Sept. 1988. p2155-2160.
Mackie, Roderick I., White, Bryan A., Isaacson, Richard E. “Gastrointestinal Microbiology”. International Thomas Publishing. New York. 1997.
Maczulak, Anne E., Karl A. Dawson, and John P. Baker. “Nitrogen Utilization in Bacterial Isolates from the Equine Cecum”. Applied and Environmental Microbiology. Dec. 1985. p. 1439-1443.
Pollitt, C.C., M. Kyaw-Tanner, K.R. French, A.W. Van Eps, J.K. Hendrikz and M. Daradka. "Equine Laminitis". 49th Annual Convention of the American Association of Equine Practitioners. 2003. New Orleans, LA.
Rowe, James B., Michael J. Lees & David W. Pethick. “Prevention of Acidosis and Laminitis Associated with Grain Feeding in Horses”. The Journal of Nutrition. 1994. Volume 124. p. 2742S-2744S.
Wright, Bob. “Equine Digestive Tract Structure and Function”. http://www.omafra.gov.on.ca/english/livestock/horses/facts/info_digest.htm September 1999.
Edited by [Tim Shaw, Mike Sullivan and Megan Tambaschi], students of Rachel Larsen