Helicobacter pylori as a causative agent of Gastric Cancer
Introduction
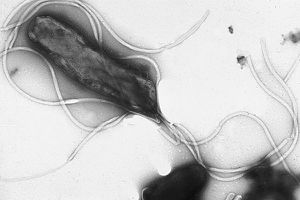
Helicobacter pylori [figure 1] is an important bacterium that colonizes in the stomachs of over two-thirds of the human population, and is especially prevalent in developing countries [1]. Although latent in the majority of infected persons, inflammation caused by H. pylori has been found to be the main factor in gastritis, peptic ulcers, and in some cases gastric cancer [2]. In fact, three-fourths of all stomach cancer cases are caused by H. pylori colonization [3]. This form of carcinogenesis is the second most prevalent cause of deaths caused by cancer, with a death count of approximately 738,000 people in 2008 [1].
In fact, H. pylori is currently the only bacterium in existence to be categorized as a carcinogen by the World Health Organization International Agency for Research on Cancer [3]. Colonization by this bacterium is a risk factor and causative agent for non-cardia gastric cancer, which affects all areas of the stomach except for the coronal area near its juncture with the esophagus [1]. In addition, infection with H. pylori is an agent for gastric mucosa-associated lymphoid tissue lymphoma (MALT), a type of blood campus [1].
There are a variety of mechanisms utilized by H. pylori that are thought to promote gastric carcinoma; overall, H. pylori causes oxidative and nitrosative stress on epithelial gastric cells, which results in both cellular and nucleic acid damage, leading to carcinogenesis [4].
Evidence for H. pylori as a causative agent of gastric adenocarcinoma is abundant, yet is only a risk factor for non-cardia gastric cancer and does not seem to have a damaging effect on gastric cardia cancer. The difference between these two carcinomas is that the former is found in the middle and lower portions of the stomach, whereas the latter presents only in the top part of the stomach near the esophagus [20]. In fact, the risk of presenting with stomach cancer is six times greater for patients infected with H. pylori than those who are uninfected [5].
Simultaneously, H. pylori infection has been found to lessen the risk of other cancers, in particular carcinoma of gastric cardia cancer and esophageal adenocarcinoma [1]. It is not known as of yet the reasoning for why H. pylori causes cancer in the caudal area of the stomach yet not in the cranial section.
The severity of disease induced by H. pylori is dependent on a variety of pathogenic factors. H. pylori [21]. First, severity is reliant on how adaptive the H. pylori is to the host environment, which is based upon genomic plasticity of the bacterium; second, the genetic resistibility and immunological strength of the host; and third, behavioral factors including smoking, poor diet, and increased consumption of sodium and iron.
Helicobacter pylori Description and Genome
Helicobacter pylori is a gram-negative proteobacterium that adheres extracellularly to the epithelial cells of the human stomach [4] [figure 2]. Acquired during infancy [4], H. pylori is typically contracted through contaminated water and food or through oral contact, especially in situations of poverty and densely populated areas [1]. The bacterium is a microaerophile and thrives in extremely acidic conditions with low levels of oxygen, such as the environment of the gastrointestinal tract [3]. The bacterium is able to survive in this type of environment through its production and use of hydrogenase that is coupled with aerobic respiration [6].
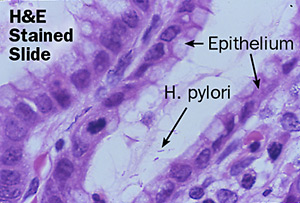
The bacterium commonly presents morphologically as a curved rod with flagella; flagella are crucial to H. pylori survival as they allow for motility through the gastric mucosal layer in order to infect the host cells [7]. Interestingly, H. pylori in the form of cocci have been observed after treatment with antibiotics [4]. It is not known as of yet whether these coccoidal structures are of significance pathogenically.
Evidence has been found to suggest that H. pylori had begun to colonize epithelial cells in humans as far back as 60,000 years ago in East Africa [8]. Mutations in the bacterial genome are distinct and indicative of human migration patterns. As of 2011, seven full genomes were sequenced, which are composed of 1.6 megabases each, and 1500 open reading frames [4]. Mutations between species have been observed in 20-30% of the genome dependent on bacterial strain, which is indicative of a high mutation rate as well as high recombination frequency [4].
Although about 80% of H. pylori colonization is benign and asymptomatic, the remaining percentage of the population infected with this bacterium experience some sort of abnormal tissue growth over time, with gastric tumors growing in less than 2% of that remaining population[4]. In varying degrees, H. pylori possess the capability to induce gastritis and low levels of gastric acid production [2]. Depletion of the organism over time within the stomach is uncommon; however, some cases have shown loss of H. pylori in situations where stomach mucous becomes unfavorable for colonization such as during metaplasia [4].
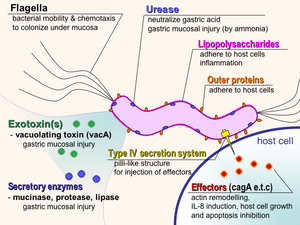
Helicobacter pylori utilizes a variety of adhesion mechanisms in order to survive within the human gastrointestinal tract. Of particular interest is the cytotoxin associated gene (CAG) pathogen island, which translates a variety of proteins that assist in depositing pathogenic products into host cells [4]. Other compounds of note are blood group antigen binding adhesin (BabA) and sialic acid binding adhesin (SabA), both of which are responsible in facilitating H. pylori binding to epithelial cells [4].
H. pylori facilitates survival within its host through the utilization of a urease enzyme which decreases the gastrointestinal acidic environment (pH 1-2) for the bacterium [4]. H. pylori flagella are particularly useful to the organism in facilitation of motion through the mucous layer that covers epithelial cells within the stomach [4].
Typically found as adhering extracellularly to epithelial cells, H. pylori has been found in some cases to be implicated in antibiotic resistance when observed intracellularly, particularly in cancers [9].
Gastric Cancer
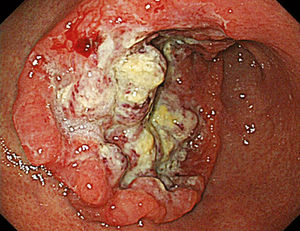
Gastric Carcinoma, Gastric Cancer, or Stomach Cancer [figure 3] normally originates in cells found on the lining of the stomach that produce mucous [10]. This form of cancer is incredibly prevalent in the developing world due to unsanitary food and water conditions, and is observed in high rates in both China and Japan. Gastric cancer is more prevalent in geriatric patients, smokers, and males; incidence rates are higher in individuals with stomach inflammation, which is commonly associated with infection of Helicobacter pylori [4],[10].
Two carcinomas of interest are adenocarcinoma and mucosal associated lymphoid tissue lymphoma (MALT) [4]. Gastric carcinoma is attributed to H. pylori colonization, and is characterized by its evolution from normal mucosa, to variant forms of gastritis, followed by abnormal cell proliferation, and ultimately late stage gastric carcinoma[2]. MALT pathogenesis occurs through proliferation of B-lymphocytes, or B-cells, in the inner gastric lining [1]. In both forms of cancer, nuclear and mitochondrial DNA damage and impairment of DNA reparation processes are observed [2].
Symptoms and Treatment
Patients infected with a pathogenic form of Helicobacter pylori may feel fatigued, bloated, nauseous, or stomach pain[11]. They may also experience heartburn, indigestion, vomiting, or drastic weight loss.
Gastritis treatment is dependent on the determined causative agent of the disease; when caused by H. pylori, gastritis is treated using antibiotics such as clarithromycin, amoxicillin, or metronidazole which kill the bacteria [11]. These medications are taken concurrently with anti-inflammatory medications that treat stomach acid, acting as proton pump inhibitors such as omeprazole, or histamine blockers which diminish acid production in the gastrointestinal tract, such as ranitidine.
Gastric cancer, as the more advanced and detrimental version of the disease inflicted by H. pylori pathogenesis, requires more aggressive treatment. Gastric carcinoma requires removal of tumors or a gastrectomy [10]. Other treatments include radiation and chemotherapy. Unfortunately, many of these treatments are not utilized until later stages of carcinogenesis, as it is difficult to diagnose gastric cancer in the early stages of the disease [12]. Gastric cancer is diagnosed with a combination of imaging and blood tests, an endoscopy, and a biopsy [12].
Process of Infection
Although 80% of colonization effects of H. pylori cohabitation are benign in almost two-thirds of the world’s population, detrimental infection rates are still incredibly common [1]. Only a small subset of H. pylori infections progresses to the stage of carcinogenesis, and the difference between manifestation severities has been postulated as being attributed to a variety of environmental factors, bacterial strain virulence and variation, and severity of inflammation by host cells [13]. In addition, researchers have discovered that probability of carcinogenesis is correlated with variants in H. pylori virulence factors, such as the CAG pathogenicity island as well as the vacuolating cytotoxin, VacA [figure 4][2].
Immune cells, such as B cells, are generally implicated in protecting the host from pathogen invasion. However, due to the fact that H. pylori colonizes past the mucosal layer within epithelial cells of the stomach lining, it is difficult for B cells to access and attack this bacterium [1]. Additionally, it has been found that H. pylori has developed mechanisms in which it is able to impede and prevent immune responses by the host locally, and therefore are able to colonize gastric tissue without the threat of an immunological response [1]. Also of interest is the implication of B cell proliferation in MALT pathogenesis, which further interferes with host immune response [2].
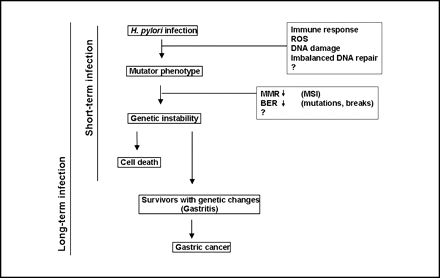
In terms of survival within the gastrointestinal tract, H. pylori is able to discharge urease, which is an enzyme that converts urea to ammonia within the host environment [1]. This chemical conversion decreases the acidity within the host’s stomach, which creates a more favorable environment for the bacterium. In relation to motility, the shape of H. pylori coupled with its utilization of flagella allows it to move easily through the stomach mucosal layer, which has a more neutral pH than stomach lumen. Ultimately, this lower acidity increases feasibility of adhesion to epithelial cells [1].
Generally, colonization of H. pylori promotes the development of gastric carcinomas through a variety of distinct processes and virulence factors [figure 5]: inducing inflammation and immune response, adhesion, DNA damage and decreased repair mechanisms, and mutation in mtDNA (mitochondrial DNA).
Adhesin
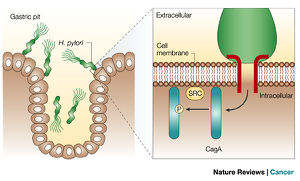
H. pylori implant a toxin into the cell lining using the cytotoxin-associated gene A (CagA) [figure 6] [14]. Recent research suggests that the toxin mutates the structure of epithelial cells in order to facilitate attachment for the bacterium. Prolonged subjection to this toxin promotes chronic inflammation as an immunological response. However, not all H. pylori are CagA-positive, suggesting the lack of CagA gene as a correlative factor for pathogenicity of the bacterium [15]. In infectious cases, CagA-positive H. pylori inhibits tumor suppressor proteins, as well as inactivates pathways that promote production of pro-inflammatory cytokines and mutations in cell polarity and defenses against invasive species [2]. Therefore, due to both the inflammatory response and suppression of protective proteins, one mechanism for carcinogenesis is proposed.
The cytotoxin-associated gene A, CagA, and vacuolating cytotoxin, VacA, are both secreted through a type IV secretion mechanism, T4SS, which is a structure that is endogenous to most bacteria [2]. During this process, a variety of molecules are discharged through a cross-membrane multi-protein channel, which is powered by ATP [19]. CagA and VacA are dispelled across the membrane for cellular interactions between the gastroepithelial lining and H. pylori, as well as for horizontal gene transfer. This functional system has been found to exhibit strong correlations with inflammation, cytoskeletal and genetic regulation, and ultimately, elevated risks for gastritis and eventually gastric carcinogenesis, due to its emission of the CagA effector protein.
DNA Repair Pathways
One pathway that creates instability within the host genome of epithelial cells by H. pylori is the inhibition of DNA repair mechanisms. This mechanism is opportunistic for the pathogen as it then is able to promote mutation in the host cells [2]. H. pylori is an interesting bacterium in that it has the ability to concurrently cause changes in the genome, such as “oxidative damage, methylation, chromosomal instability, microsatellite instability and mutations.”[2] In this case, not only are immunological defenses inhibited, but the antioxidant defenses of epithelial gastric cells are hindered as well, indicating that H. pylori are able to avoid the onset of reactive oxygen species which would destroy the bacterium and would not allow it to survive due to its microaerophilic characteristic. As a result H. pylori is able to induce deregulation of DNA repair pathways as well as breed genomic instability in host DNA, thus creating a more beneficial host environment for the bacterium. As a result, these mechanisms aid in the causative agents of both acceleration of cell death and defense against further growth of host cells.
H. pylori also causes mutations in the mitochondrial DNA (mtDNA) of the host cells [2]. Various studies have observed that in a wide range of cancers mtDNA mutations are correlated with carcinogenesis development, although not with tumor amplification [2]. These studies have also discovered that the mitochondrial genome in host cells is extremely fragile and vulnerable to colonization, infection, and mutations by bacteria, as researchers were able to induce changes in the mitochondrial DNA with the introduction of H. pylori both in vivo and in vitro. Research suggests that H. pylori induces these mutations for bacterium transformation and survival mechanisms (see Figure 5).
An important aspect of these DNA repair pathways is the regulation of varying mutations that in some cases can inhibit tumor suppressor genes and promote development of oncogenes, both of which contribute to carcinogenesis through the inactivation of the mismatch repair (MMR) pathway [2]. This inhibitory mechanism occurs at an early point in H. pylori infection and eventually causes neoplasm formation and reproduction.
Inflammation
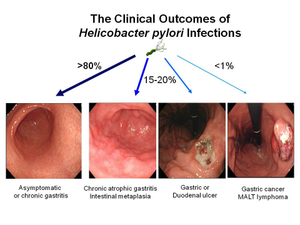
Colonization and infection of H. pylori results in an inflammatory response that is aided in immunological defense by neutrophils, lymphocytes, plasma cells, and macrophages [2]. Recent research has elucidated that this response may actually be a factor in the eventual severity of the infection [figure 7], as the severity of inflammation may actually lead to increased risk of carcinogenesis because of DNA damage, which elevates proliferation and apoptosis rates as well as promoting development of growth factors [2]. This inflammation is pertinent as it especially relates to the swelling it causes within not only the stomach but also the duodenum, and induces the development of cytokines which act as inflammatory factors in the form of tumor necrosis factor alpha (TNF-α), interleukins 1 and 6, and chemokines, all of which are thought to be related to the promotion of carcinogenetic activity as a direct result of H. pylori colonization [3].
Overall, H. pylori colonization is correlated with elevated rates of cellular apoptosis [2]. When apoptosis caused by this bacterium does not occur concurrently with cell proliferation, gastric ulcers present with more common frequency and increased levels of inflammation are observed, eventually culminating in gastric atrophy.
Recent Research
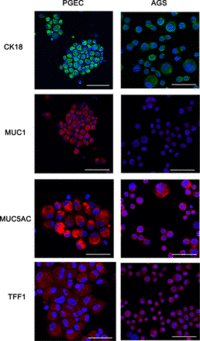
Recent studies from the Centre Hospitalier Universitaire de Poitiers in Paris have attempted to characterize and analyze inflammation patterns that occur as a result of infection with H. pylori [22]. Translocation performed by H. pyloriof the virulence protein CagA into host epithelial cells induces inflammation through signaling of cytokines and chemokines. Researchers isolated and analyzed H. pylori inoculated primary gastric epithelial cells (PGEC) and AGS cells (adenocarcinoma cells that lack toll-like receptors and can therefore not signal the innate immune response required). Reverse transcription-quantitative PCR analysis was then performed in order to elucidate the inflammatory profile of both types of cells. Markers were expressed that demonstrated significantly higher levels of PGEC mRNA of chemokines and cytokines CXCL-1, CXCL-2, CXCL-3, CXCL-5, CXCL-8, CXCL-20, and TNF-α, in comparison with the panel found in AGS cells (figure 8). They discovered that this was attributable to CAG pathogenicity island substrate-independent virulence factors, which are responsible for inducing preliminary inflammation in gastroepithelial cells.
Ultimately, this research showed that the chemokines that were activated during induction with H. pylori into gastroepithelial cells are essential for signaling both dendritic cells and lymphocytes to activate an immunological response in the form of inflammation. Interestingly, this signaling pathway has been found to be directly associated with the translocation of the CagA cytotoxin into gastroepithelial cells, which is accredited to the proper functioning of the type IV secretion mechanism (T4SS).
Future Work
Although vast amounts of knowledge are understood about the mechanisms by which Helicobacter pylori is able to inhabit and infect human gastric epithelial cells and in virulent strains as the main risk factor in inducing gastric carcinoma, virtually no information is known about the prevention of not only pathogenic strains but also about latent strains of the bacterium. As of now, one study that was completed in China demonstrated that small doses of preventative antibiotic treatment in order to eliminate H. pylori from patients actually decreased the incidence of gastric cancer [16]. In fact, over 15 years after treatment with these antibiotics, incidence rates of gastric carcinoma surprisingly decreased by over 40 percent. However, other than preventative use of antibiotics, which was not completely effective and could potentially have other long term risks, virtually nothing is known as to how to prevent this bacterium from colonizing within over three-fourths of the world’s population.
Another important idea that is vital to understanding the mechanism of H. pylori as it relates to gastritis and varying degrees of carcinogenesis within the gastrointestinal tract, is the reasoning behind why the bacterium is correlated with high prevalence rates of MALT lymphoma and gastric adenocarcinoma and at the same time is also associated with a decreased risk of esophageal adenocarcinoma and cancer of the upper stomach. In Sweden, researchers have discovered that patients infected with H. pylori were one-third less likely to be at risk for esophageal adenocarcinoma in comparison to individuals who presented without colonization of H. pylori [17]. Some believe that this is a direct result of the chronic inflammatory response induced by H. pylori, which promotes expression of cytokine interleukin-1 which predisposes epithelial cells to cancer [18]. This contradiction is incredibly fascinating and also pertinent to both the microbial and clinical realms, and its mechanism will be more closely studied in the future.
Future research will hopefully also include investigation on not only ways to preventatively inhibit the damaging activity of Helicobacter pylori in addition to preventions of cancer, but would also promote research on detection techniques for virulent genes such as CagA and discovery and prevention of early stage gastric carcinomas.
References
Edited by Alexandra Kruse, student of Joan Slonczewski for BIOL 238 Microbiology, 2015, Kenyon College.
