Middle East respiratory syndrome coronavirus


Etiology/Bacteriology
Taxonomy
Scientific name: Middle East respiratory syndrome coronavirus
Common name: MERS-CoV
Viruses; | Group = ssRNA positive-strand viruses | Order = Nidovirales | Family = Coronaviridae | Subfamily = Coronavirinae | Genus = Betacoronavirus | Species = unclassified Betacoronavirus
Description
Middle East respiratory syndrome coronavirus (MERS-CoV) infects the respiratory system, causing the severe acute illness Middle East respiratory syndrome also known as MERS. MERS is second to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) as a highly pathogenic coronavirus. [4] The virus falls under lineage C of the genus Betacoronavirus based on its genome. Though the symptoms are often identical to SARS, the two coronaviruses differ in their genomic sequence with MERS-CoV being more closely related to the bat-derived HKU4 and HKU5 coronaviruses. [5] It was first identified in Jiddah, Saudi Arabia in 2012 and soon afterward seen in other Middle Eastern countries including Jordan, Qatar, and the United Arab Emirates. In other cases, it has been observed in countries including France, Germany, Italy, the United Arab Emirates, China, Malaysia, South Korea, and the United States. All confirmed cases are linked to the Arabian Peninsula. Coronaviruses are characterized by their crown-like, coronal glycoprotein spikes on their surface. They have enveloped virions that measure approximately 120nm in diameter with a nucleocapsid made up of viral nucleic acids and are helical in shape. The genome consists of a single strand of RNA. [1]
Pathogenesis
Transmission
MERS-CoV is a zoonotic virus that is transmitted from animals to humans. Though not fully known, the virus is believed to have originated in bats that were then transmitted to camels. [2] So far, there have been no published reports of autopsies of fatal MERS-CoV cases. Therefore, the pathology of MERS-CoV contains some speculation. Transmission is reported to take place during both the symptomatic and incubation stages. [13] Transmission to humans has been linked to camels as the major animal source since strains of MERS-CoV found in humans have been matched with strains found from camels from the Middle East. However, the mechanism of how the virus transfers to humans from animals is not fully understood. The virus appears to not easily pass from human to human but is seen more frequently when there is close contact such as in a hospital setting. The virus is spread from humans to humans by respiratory secretions such as coughing or drinking after the infected. [2]
The virus mainly circulates throughout the Arabian Peninsula where >85% of the cases have been reported. However, there have been several reports outside of the Middle East, where travelers have contracted the infection during their visit to the Middle East and then brought back to their home country.
Currently, the outbreak in Republic of Korea in 2015 has been the largest outbreak outside of the Middle East. [3]
Incubation/Colonization
CD26 (diptptidyl peptidase 4, DPP4) is the cellular receptor for MERS-CoV4. When MERS-CoV spike (S) protein interacts with CD26, viral attachment to host cells occurs with virus to cell fusion which starts infection. The MERS-CoV protein would cleave the host cells into S1 and S2 subunits. S1 engages receptor 4 whereas S2 mediates membrane fusion. [5]
Epidemiology
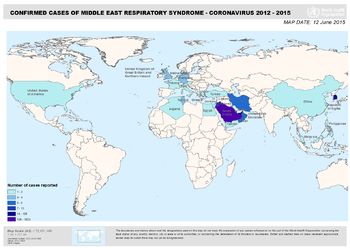
The MERS coronavirus seems to be closely related to European and African bats particularly relating to the Vespertilionidae and Nycteridae families, making the infection zoonotic with the possibility of human-to-human transmission. [7] The first report of the disease was from a man in Saudi Arabia by a virologist, Dr. Ali Mohamed Zaki, in September of 2012 but it was later known that earlier cases of MERS had occurred in Jordan in April. [6] Since 2012, MERS-CoV has spread to nearby countries of the Arabian Peninsula with all cases in other countries linking back to the Middle East. In 2012, Germany and the United Kingdom had reports of MERS as well. In 2013, France, Germany, Italy, Kuwait, Oman, Qatar, Tunisia, and the Arab Emirates were added to the list. In 2014, Algeria, Austria, Greece, Turkey, Lebanon, Malaysia, the Netherlands, and Yemen showed MERS-CoV infected patients. Currently in 2015, Iran, Philippines, Thailand, and China all inhabited infected persons with Republic of Korea harboring the largest outbreak outside of the Middle East. Most other infected countries outside of the Arabian Peninsula have not resulted in a high number of contagion. The exact animal-to-human transmission as well as human-to-human transmission mechanism is not yet fully understood but all reported cases have been linked back to originate from the Middle East. [3]
Virulence Factors
MERS-CoV is able to counteract the early innate immune response. [9] Type I interferon (IFN) is essential in the role in antiviral innate immunity in which MERS-CoV is efficiently able to inhibit the activation of the Type I interferon response. [10] In addition, the spike protein is a virulence factor in many different coronaviruses. [11]
Clinical features
Symptoms
Persons who have contracted MERS-CoV can be asymptomatic or have mild respiratory symptoms. Many times, patients show severe acute respiratory symptoms that can lead to death. Typically, these patients show symptoms of fever, cough, shortness of breath as well as diarrhea and vomiting.
Severe illness can lead to respiratory failure that requires mechanical ventilation. More severe complications have been seen to result in pneumonia and kidney failure. The virus seems to cause more severe disease and higher risk of death with those who are older or have an already weakened immune system though persons of all ages can become infected. [3] This may be the case since older patients or patients with pre-existing co-morbid conditions may not have the immune system to combat the viral infection with type I IFN host responses. Aging and co-morbid conditions are known to have negative effects on the ability to mount a strong type I IFN response. [12]
Morbidity and Mortality
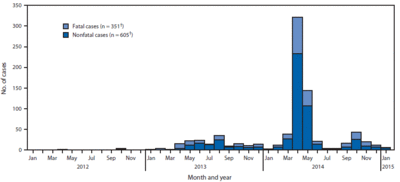
As of January 23, 2015, WHO has confirmed 956 confirmed cases of MERS-CoV infection with 351 deaths. Therefore, approximately 36% of all reported patients result in mortality.
In the most recent outbreak in the Republic of Korea, a total of 186 cases were confirmed with 36 deaths making it the largest outbreak outside of the Middle East. [3]
Diagnosis
Diagnosis is based off of symptoms shown such as coughing and shortness of breath within 14 days of traveling directly to an affected country or near the Arabian Peninsula, or if there was any close contact with someone showing these symptoms who has recently been exposed to countries affected by MERS-CoV. Those who have also been in a healthcare facility in the Republic of Korea and have generated symptoms should also contact a healthcare provider. Persons who have had direct contact with camel in any form especially near the Arabian Peninsula should also be concerned. Once symptoms have been confirmed, molecular tests can be done to diagnose an active infection. Real-time reverse-transcription polymerase chain reaction (rRT-PCR) assay tests are used to detect viral RNA by using respiratory samples. Typically, several specimens may be tested using the rRT-PCR test for confirmation. [2]
Post Infection Studies
Serology tests are also used for investigational purposes and not for diagnosis. Serology testing assesses for previous infection by observing antibodies. Serology testing includes taking a sample of blood and searching for MERS-CoV antibodies for those who have been infected and developed immunological memory. Three tests are included for serology testing of MERS-CoV. The first is a screening test by enzyme-linked immunosorbent assay (ELISA), which detects for specific antigens. This test looks for specific antibodies binding to a viral protein. The second test is for confirmation using immunofluorescence assay (IFA), which uses fluorescent-labeled antibodies to detect specific target antigens by taking a blood sample and seeing if specific antibodies attach to virus-infected cells on a glass slide. Then a fluorescent-labeled antibody compound is used to see if binding occurred by the presence of a glowing green color, which can be seen under a special microscope. The third is a slower but more complete confirmation using the neutralizing antibody assay test. This test is highly specific and measures antibodies that can neutralize virus. A compilation of these tests are done to confirm MERS-CoV infection. These serology tests are used to test individuals who may have had the virus but showed no symptoms. In this way, scientists can further learn about how the virus is transmitted. [2]
Prevention and Treatment
Currently, there are no vaccines for prevention or specific antiviral treatments for cures. However, individuals with MERS-CoV infection can seek medical attention to relieve symptoms and receive support for boosted organ functions. The CDC recommends avoiding personal contact and frequent hand washing in order to prevent respiratory illnesses.[2] Also for precaution, persons should avoid contact with camels especially when traveling to the Middle East. [3]
Damage Response Framework
The incubation period for MERS is usually about 5 or 6 days but can range from 2-14 days depending on the case. Most infected patients run through the course of their symptoms which are different from person to person. After there are no signs of symptoms, patients must wait 14 days until returning to normal activity. Due to the limited knowledge of MERS-CoV and the fact that the virus is so new, there has been no definite timeline of the immune response. [2]
Concerns
Although the MERS-CoV outbreak is more restricted in terms of transmission when compared to SARS, there is speculation that there may be future adaptation in which there is an increase in human-to-human transmission. With limited knowledge of the pathogenesis of virus, it is possible that there may also be an adaptation to the virulence factors, causing the disease to become more severe. With the virus being recently discovered, there is not enough research that has been done, therefore, it is difficult to predict the pathology of MERS-CoV infection. Currently there have been no reports of autopsies on fatal cases and not enough studies in animal models that could provide information of its characteristics. There are also many pre-existing health conditions that make it difficult to isolate definite answers from research done on patients with MERS-CoV. [8]
References
1 "MERS." Encyclopaedia Britannica. Britannica Academic. Encyclopædia Britannica Inc., 2015. Web. 22 Jul. 2015. <http://academic.eb.com/EBchecked/topic/1949834/MERS>
2 Centers for Disease Control and Prevention, Middle East Respiratory Syndrome (MERS) <http://www.cdc.gov/coronavirus/mers/index.html>
3 "Middle East Respiratory Syndrome Coronavirus (MERS-CoV)." WHO. N.p., n.d. Web. 25 July 2015. <http://www.who.int/mediacentre/factsheets/mers-cov/en/>
4 Lu, Guangwen, et al. "Molecular Basis Of Binding Between Novel Human Coronavirus MERS-Cov And Its Receptor CD26." Nature 500.7461 (2013): 227-231. Academic Search Elite. Web. 25 July 2015. <http://web.a.ebscohost.com.ezproxy.lib.ou.edu/ehost/pdfviewer/pdfviewer?sid=6237c9b9-e0bf-4e3e-93ab-af58ed5b76b5%40sessionmgr4002&vid=2&hid=4204>
5 Lu G, Liu D (2012) SARS-like virus in the Middle East: a truly bat-related coronavirus causing human diseases. Protein Cell 3: 803-805.<http://link.springer.com.ezproxy.lib.ou.edu/article/10.1007/s13238-012-2811-1>
6 Ali Mohamed Zaki; Sander van Boheemen; Theo M. Bestebroer; Albert D.M.E. Osterhaus; Ron A.M. Fouchier (8 November 2012). "Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia" (PDF). New England Journal of Medicine 367 (19): 1814–20. <http://www.virology-bonn.de/fileadmin/user_upload/_temp_/Zaki_et_al.pdf.>
7 J. Virol. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group July 2013 vol. 87 no. 147790-7792 <http://jvi.asm.org/content/87/14/7790.short#ref-list-1>
8 van den Brand, J. M., Smits, S. L. and Haagmans, B. L. (2015), Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol., 235: 175–184. doi: 10.1002/path.4458 <http://onlinelibrary.wiley.com.ezproxy.lib.ou.edu/doi/10.1002/path.4458/full>
9 Perlman S, Dandekar AA. 2005. Immunopathogenesis of coronavirus infections: implications for SARS.Nat. Rev. Immunol. 5:917–927 < http://www.ncbi.nlm.nih.gov/pubmed/16322745>
10 Zielecki F, Weber M, Eickmann M, Spiegelberg L, Zaki AM, Matrosovich M, Becker S, Weber F. 2013. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J. Virol. 87:5300–5304 < http://www.ncbi.nlm.nih.gov/pubmed/23449793>
11 "SARS Reference | Virology." SARS Reference | Virology. N.p., n.d. Web. 28 July 2015. < http://www.sarsreference.com/sarsref/virol.htm>
12 Stout-Delgado HW, Yang X, Walker WE, et al. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol 2008; 181: 6747–6756. < http://www.ncbi.nlm.nih.gov.ezproxy.lib.ou.edu/pubmed/18981092>
13 Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13: 752–761. < http://www.ncbi.nlm.nih.gov.ezproxy.lib.ou.edu/pubmed/23891402>
Created by Hae Cha, student of Tyrrell Conway at the University of Oklahoma.
