Modern Treatments for Hepatitis C Virus
by Devon Donohue
Introduction
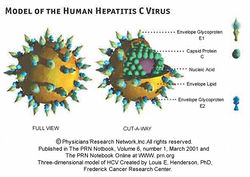
Hepatitis C is a human disease caused by the virus Hepatitis C Virus (also known as HCV). According to the CDC, about 16,000 acute hepatitis C cases are reported each year. Whilst some of these acute infections can be entirely removed from the individual, many patients develop chronic Hepatitis C. About 75-85% of individuals with acute Hepatitis C will develop chronic Hepatitis C. In the US, it is estimated that about 3.2 million people suffer from chronic Hepatitis C virus [5]. Worldwide, it is estimated that anywhere from 130 to 150 million people are living with chronic Hepatitis C infections. [6] HCV (see Figure 1) is an icosahedral virus of the flaviviridae family, genus hepacivirus. It can be transmitted from person to person via contact with contaminated blood, or less commonly by sexual contact with an affected person. The virus is mainly spread by sharing of contaminated drug paraphernalia (such as needles), blood transfusions, accidental needle sticks, or sexual intercourse. To test whether or not someone has been exposed to Hepatitis C, antibodies in the patient are tested to see if they are fighting a Hepatitis C infection. This immune response is the best way to confirm that a person has contracted Hepatitis C. The structure of the virus (Figure 2) is optimized for introduction into human cells. The virus has an outermost layer of lipid membrane and an inner layer of icosahedral protein which contains the positive sense coding RNA that is the genetic material for this virus. The outer lipid membrane also includes two glycoproteins known as E1 and E2. In order to infect human cells, the virion will merge its lipid membrane with that of the human cell, and use the inner protein layer to inject the RNA into the nucleus. From there, the virus will force the cell to make many replicates, spreading the virus even more throughout the person’s body.
Hepatitis C, as its name suggests, is one of five different viruses that causes Hepatitis in humans. Hepatitis A is an acute infection that is easily transmissible through water or food, but is also easily cleared by the immune system without additional treatment. There is also an effective vaccine that prevents Hepatitis A that is readily available. Hepatitis B can cause chronic hepatitis, but cannot be spread as easily as Hepatitis A. It can be treated using an aggressive treatment similar to Hepatitis C virus. Luckily, there is also an effective vaccine available for Hepatitis B as well. Hepatitis D and Hepatitis E are not as common as the other three viruses, although Hepatitis E can also be spread by water supply and is therefore a health risk [7]. Hepatitis C causes severe, chronic liver disease in infected individuals. The cirrhosis of the liver often becomes so serious that liver transplant becomes necessary in patients who have had hepatitis C without treatment. Adding to the danger of this infection, many individuals are asymptomatic until liver damage becomes extremely severe. This can be catastrophic because these individuals can be spreading the virus without ever receiving treatment or knowing that they are carriers for this infection. The virus mainly replicates in the hepatic liver cells, as well as some of mononuclear red blood cells. These blood cells are essential for immune response; so many individuals with Hepatitis C have immunodeficiency symptoms as well as liver failure. Currently there is no vaccine for this virus, thus immediate detection and early treatment is essential to the prevention of newly infected individuals, as well as patient survival. In the last few years, the treatment options for Hepatitis C have improved greatly, with several new drugs reaching approval by the FDA and several others in states of testing. The main goal of hepatitis medication is to lower the viral load and to enable the body to fight against the virus effectively, reducing the risk to others and decreasing the risk of sustaining permanent liver damage. In the last ten years, the most effective treatment option was to take doses of peginterferon-alpha plus ribavirin. The effectiveness of this treatment will be discussed in this article, as well as some newer drug regimens that have been developed over the past year. One of these drugs, named Sofosbuvir, was recently approved by the FDA to treat Hepatitis C. The other drug, called Simeprevir, was also approved in the last year for treatment of Hepatitis C, using a different biochemical response to that of Sofosbuvir. These two drugs represent the forefront of biomedical research to cure a dangerous and debilitating disease.
Interferon Treatment: Peginterferon alpha 2a plus ribavirin
A Peginterferon is a type of interferon, which is a protein that helps create an immune response to viruses in humans. More specifically, interferons are cytokines produced by lymphocytes that assist cells in communicating in order to rid the body of viruses, and can coordinate the release of macrophages and T-lymphocytes in order to destroy virions. In addition, interferons can protect uninfected cells from becoming infected, and interfere with viral replication inside the host cells. Peginterferon is a special type of interferon that is “pegylated”, meaning that in addition to the protein structure of an interferon it also has a polyethylene glycol group added to it as well. This group is intended to give the interferon a greater half-life than a non pegylated interferon. The designation alpha refers to its classification as an IFN-I, which designates interferons based on their activity. Although the exact mechanism of peginterferon alpha 2a is not known, recent studies have shown that peginterferons activate genes that have a wide variety of antiviral response, which helps to explain why it is such a good choice to use on HCV patients. It should be noted that as of now, peginterferon alpha 2a is only effective on a few strains of HCV. HCV strains vary greatly, and frequently morph so quickly that within a few generations an HCV strain can manifest like an entirely new species. Interferon treatment for HCV is not perfect, however. Peginterferons require the addition of ribavirin in order to be fully effective as a treatment option. In addition, peginterferon treatment comes with severe side effects, including seizures, temporary blindness, hair loss, or worsening of preexisting conditions such as high blood pressure or immune disorders such as lupus.
Ribavirin in itself is not a complete treatment for Hepatitis C. This drug was first discovered in the 1970’s as a potential treatment for respiratory synctitial virus, but was found later to have greater effect against flaviviruses such as HCV. However, studies showed that it did not reduce symptoms of HCV until it was combined with interferon treatment. When combined with interferon treatment, this regimen was shown to significantly increase the number of patients who rid themselves of the virus, and to decrease relapses post-treatment. Now, ribavirin is an integral part of the treatment plan for HCV. The mechanism by which ribavirin works is not entirely understood. It is believed that ribavirin inhibits GTP production, which the virion needs in order to assemble its viral RNA. Similarly to peginterferon, ribavirin has many serious side effects conditional upon its use. Ribavirin has been shown to cause serious birth defects in pregnant women, and can worsen many pre-existing conditions.
A study completed in 2000 showed that peginterferon alpha 2a plus ribavirin had a much greater immune response in patients than interferon alpha 2b plus ribavirin. In addition, the study (Manns et al. 2001) showed that higher doses of peginterferon alpha 2a combined with higher doses of ribavirin had the greatest success in clearing the viral load in patients. The sustained viral response of the patients taking peginterferon alpha was an average 44% of patients, versus about 40% of patients taking interferon alpha 2b.
In addition to adverse side effects, these two drugs also are inconvenient for patients in other ways. Peginterferon is a subcutaneous medication, meaning it must be injected into the bloodstream rather than be taken orally. In addition, the standard treatment regimen for peginterferon and ribavirin treatment is 48 weeks of treatment, after which it takes an additional 24 weeks to demonstrate blood clear of viral RNA.
Sofosbuvir
Sofosbuvir is a relatively new drug regimen approved by the FDA in November of 2013 in order to treat HCV. As opposed to peginterferon treatment, Sofosbuvir is an oral medication that only needs to be taken once a day. It descends from intermediary medications such as Boceprevir and telaprevir. These two medications are HCV protease inhibitors, and are also taken orally. Unlike interferon treatment, these medications act directly on the hepatitis virions and work much more effectively to clear the patient of viral activity when combined with peginterferon and ribavirin treatment. However, this three drug regimen can become cumbersome and unfeasible for those who cannot afford three expensive medications. In addition, both of these medications have an extremely short half-life which necessitates the administration of each drug three times a day.
[[Image: Chemical_Structure_of_solvadi.jpeg|thumb|300px|right|Figure 3.Sofosbuvir is a revolutionary drug that, when taken in combination with other HCV medication can greatly reduce the length of treatment and increase the chance of ridding the body of the virus. This medication is the first hepatitis treatment to be taken independent of peginterferon treatment. It can be taken in combination with interferon treatment, but can be just as effective against HCV when taken with ribavirin. Studies such as the clinical trial review in May 2013 by Lawitz et al. [8] showed that Sofosbuvir will cause an extremely high SVR in 90% of patients that did not respond to interferon treatment. This drug is also show to be more effective on the different genotypes of HCV, whereas interferon treatment is most effective only on genotype 1 infections. In addition, Sofosbuvir can be administered to patients with an HIV co-infection, whereas many peginterferons are contraindicated for patients with HIV infections. Sofosbuvir works directly on the HCV virions as an inhibitor of the RNA dependent RNA polymerase. This means that it effectively prevents virions from replicating in liver cells and blood cells. In addition, it also metabolizes to form uridine analog triphosphate. This molecule incorporates itself into viral RNA and acts as a termination sequence for the RNA, which will also effectively inhibit the replication of virions. Sofosbuvir can even work on strains of HCV which are resistant to protease inhibitors such as Bocoprevir and Telaprevir. Due to this revolutionary methodology, treatment regimens using Sofosbuvir are only 12 weeks on average, and the recommended daily dose is only 400 mg. This dosage comes in the form of a daily oral pill, which is much more convenient than injections of peginterferons. The side effects of this drug are much less severe than those using interferon treatments as well, with patients experiencing mostly fatigue and headaches in response to this medication. Sofosbuvir can also be taken in conjunction with Simeprevir in order to be most effective. The drug is metabolized in the liver and excreted in the urine, and is relatively safe for most patients, including those with additional chronic conditions such as renal failure or hypertension. This revolutionary drug will greatly aid the plight of patients experiencing HCV as a fatal or near-fatal disease.
Simeprevir
Simeprevir is a new HCV drug that was also recently approved in late 2013. It is another drug that acts directly on the HCV. Simeprevir acts as a protease inhibitor for the hepatitis C virus, and by inhibiting the protease NS3/4A it will work to prevent replication of the virus in liver and blood cells in human patients. Simeprevir, unlike Sofosbuvir, cannot be used alone to treat HCV infection. It must be used in conjunction with interferon treatment. Therefore, it cannot be administered to patients who are unable to tolerate interferon treatment. Simeprevir also cannot be administered to patients who have a co-infection of HIV. Since this represents a significant portion of HCV patients, this is an important distinction to make in the different treatment options available to patients. Compared with the interferon plus ribavirin regimen, patients who used all three medications were shown to have 80% sustained viral response, which would generally lead to a complete absence of viral RNA in subsequent blood tests. Interferon plus ribavirin treatment generally had about a 50% sustained viral response in the best case scenarios. Simeprevir can also reduce the time necessary for treatment, just as Sofosbuvir does.
One negative aspect to Simeprevir is that is has particularly severe side effects demonstrated in patients of East Asian heritage. The side effects that appear to be the most severe are rashes and photosensitivity. These side effects were apparently so severe in some test patients observed in the study by Hussar and Jin (2014) that they required prolonged hospitalization. Therefore, patients of East Asian descent may be contraindicated for this medication. Simeprevir’s effectiveness is increased when taken concurrently with food. The three drug regimen should be taken for 12 weeks, but can be taken for 24 weeks if additional treatment is required. Treatment doses for Simeprevir are lower than that of Sofosbuvir, with the dosage per capsule at 150 mg. Simeprevir is also metabolized in the liver, and is excreted through fecal matter.
Future Treatments
These two options for the treatment of hepatitis C virus are extremely promising, and will make a large impact on the lives of those living with this terrible affliction. In promoting research to develop yet more effective medications, researchers will simultaneously reduce the number of patients who require liver transplants, and increase the life expectancy as well as the quality of life for many. It should be noted as well that currently only wealthy patients in developed countries have access to these life-saving medications. Interferon treatments are extremely costly, and they require regular access to a health care provider in order to be administered. The two new treatments, which are taken orally, remove the need for patients to have a health provider nearby. Since many people who are affected by this disease have neither the money nor access to a dedicated healthcare provider, these new treatments will help to save many lives, especially as they become less expensive for the general public. Future research will no doubt also be focused on providing an effective vaccination for this virus, as there are already vaccines for hepatitis A and B.
References
1. Hussar, D., & Jin, Z. (2014). New drugs: simeprevir, sofosbuvir, and dolutegravir sodium. Journal of The American Pharmacists Association: Japha, 54(2), 202-207. doi:10.1331/JAPhA.2014.14513
2. Manns, M. et al. (2000). Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. The Lancet: Vol. 358, 958-965
3. M.L. Shiffman (ed.), (2012) Chronic Hepatitis C Virus: Advances in Treatment, Promise for the Future,p. 115-123
4. Slonczewski, J.L and Foster, J.W., 2014, Microbiology: An Evolving Science, W.W. Norton and Co, New York, NY.
5. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368(20):1878-87.
6. Hepatitis C Information for the Public [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; c2014 [cited 2014 April 23rd]. Available from: http://www.cdc.gov/Hepatitis/C/index.htm.
7. Guidelines for the screening, care, and treatment of persons with Hepatitis C. WHO: Produced April 2014. Available from: http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1&ua=1
8. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, et al. 2014. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370(16):1483-93.