Nitrogen Cycle
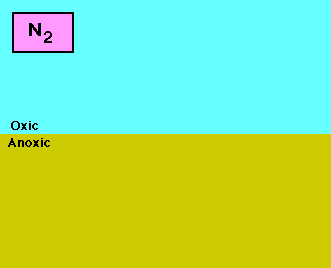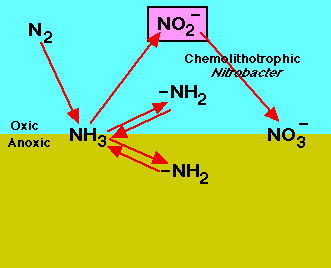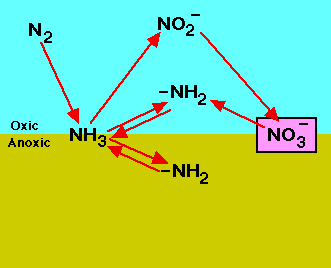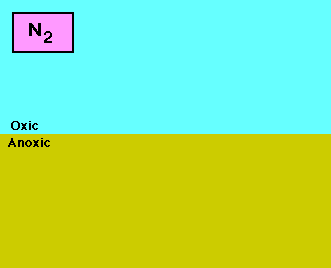
Nitrogen is one of the most abundant elements on Earth - it makes up 78% of the atmosphere, 16% of all protein diets[1], and is present at a concentration of 7.5 kilograms for every 250 kilograms of human feces[2].
The different forms of nitrogen undergo various chemical and physical transformations that are all equally critical to the global nitrogen cycle. Over the last few decades, excessive fertilizer and fossil fuel usage have lead to serious environmental problems, which have increased disease and pollution. Some of these issues include nitrate-contaminated groundwater, eutrophication, and increased production of carbon dioxide, methane, and other harmful greenhouse gases in the carbon cycle[3].
Accordingly, researchers have focused on balancing the cost to the benefits of human activities. As part of the global nitrogen cycle, soil is heavily studied not only because a significant amount of nitrogen is stored in the soil[3], but also because the increasing human population demands more nitrogen for food production.
In soil, most of the chemical transformations of nitrogen are due to microbial activities, such as nitrogen fixation, nitrification, immobilization, and denitrification. Learning how organisms behave in soil is critical for humans to understand the complex nitrogen cycle.
Nitrogen cycle processes
Natural Soil Nitrogen Cycle
Dinitrogen is an essential element for the nitrogen cycle. The symbiotic microbes fix the dinitrogen (N2) into volatile ammonia, NH3, by the process of nitrogen fixation. The ammonia is further protonated to ammonium, NH4+, which is uptaken by plants to manufacture amino acids for growth. When plants decompose, the organic molecules present in the plant residues become a source of nitrogen and other nutrients to soil microbes. The microbes conduct mineralization (ammonification) to utilize the organic molecules as electron donors, acquiring energy and producing ammonium. In contrast, the transformation of organic nitrogen to ammonium is reversible through immobilization (assimilation) when the carbon to nitrogen ratio (C/N ratio) is high[4]. Ammonium can also undergo nitrification to produce NO3- in an aerobic environments. The NO3- can be utilized by plants or by other organisms in anaerobic environments as an electron acceptor, can leach out by dissolving in water, or be reduced to dinitrogen gas via denitrification processes in anaerobic conditions. During the denitrification and nitrification processes nitrous oxide, N2O, is produced and escapes into atmosphere.
Anthropogenic Influences on the Soil Nitrogen Cycle
One major influence of human input is reduced biological nitrogen fixation. With excessive ammonium input, plants no longer need the symbiotic microbes to provide ammonium. As a result, the degree of symbiosis will be diminished. Furthermore, the excessive ammonium provides nutrients for nitrifiers to produce nitrate, causing an increase in the amount of leached nitrate. Another negative outcome of anthropogenic inputs is the increased emission of nitrous oxide into the atmosphere due to increased nitrification and denitrification from excessive ammonium inputs[3].
Nitrogen fixation
Prokaryotes(bacteria) are responsible for the nitrogen fixation inside the soil. They mostly establish associative relationships with leguminous plants and other plant species[5]. Yet, there are free-living bacteria like Azotobacter[6]. All of the prokaryotes that are capable of nitrogen fixation have nitrogenase, which can fix nitrogen into ammonia. Many prokaryotes share the same enzyme complex through horizontal gene transfer via plasmids or evolutionary events[6].
Nitrogen Fixation Mechanism
The overall reaction catalyzed by nitrogenase is N2 + 8H+ + 8e- + 16 ATP = 2NH3 + H2 + 16ADP + 16 Pi[7]. In the process, nitrogen is used to replace a pair of hydrogen molecules on the nitrogenase complex, which has been heavily reduced along with protonation. The rest of the H+ will bond to the nitrogen and reform the structure of the molecule. Then, H+ continues to be added to the complex and binds with an NH group to form NH3. Each complex is able to produce two NH3 molecules in each cycle of this process[7]. Once NH3 leaves the nitrogenase complex, it will be rapidly protonated to NH4+.
Mechanisms of Creating Anaerobic Environment
Nitrogenase are inhibited by O2[8], thus anaerobic environments are required for conducting nitrogen fixation. Free-living bacteria like Azotobacter have a couple of methods to overcome the aerobic condition. Azotobacter has a very high rate of respiratory metabolism so that it can keep the concentration of oxygen low within the cell in order to protect the nitrogenase enzyme. The second method adapted by cultured Azotobacter and Rhizobium species is to excrete large amounts of extracellular polymers that reduce the diffusion of oxygen. Leghemoglobin is a molecule in legume modules that are used to trap oxygen via chemical mechanisms that are similar to those used in hemoglobin[8]. Other microorganisms, such as the cyanobacteria Nostoc, form special structures with thick cell walls that exclude oxygen and allow the cell to provide fixed nitrogen to its neighbors. Other cyanobacteria species have specific cells within their body that are only used for nitrogen fixation. In these cells, there is only photosystem I for ATP generation, whereas other cells have both photosystem I and II, which generate oxygen. Alternative nitrogenase that are not deactivated by oxygen do exist but are found in a relatively small number of organisms[8].
Key Microorganisms
The portion of nitrogen fixed by prokaryotes make up the vast majority of all biologically active nitrogen on the planet. Diazotrophs are a category of all organisms that are capable of fixing nitrogen[9]. Diazotrophs either coexist with plants via symbiosis or have the ability to live freely inside the soil. Azorhizobium, Bradyrhizobium, Rhizobium, Bradyrhizobium, Sinorhizobium, Mesorhizobium, and Allorhizobium are genera that have symbiotic relationships with leguminous plants [5]. Diazotrophs modify leguminous root hairs into nodules to create an anaerobic environment and to provide an energy source in the form of organic carbon. In return, bacteria provide ammonium to the plant as a component for plant growth. There are also other genera that have different relationships with plants. For instance, Frankia has actinorrhizal symbiosis with a tree genus known as Casuarina[5]. There are some genera, like Azotobacter, that live freely in aerobic environments. and some, like Desulfovibrio, that live freely in anaerobic environments[8]. A more unique example of a symbiotic relationship is that of lichens. Cyanobacteria, which is a photoautotrophic genus that fixes nitrogen, are protected by fungal hyphae. In return, the fungi receive a critical nutrient source in the form of ammonium [10].
Nitrogen Ammonification
Net nitrogen mineralization is commonly defined as the sum of concurrent ammonium production and consumption processes[9]. However, in recent years, it has been shown that mineralization also is driven by depolymerization of nitrogen containing polymers available to use both by plants and microorganisms[11], and thus it is more correct to call the process ammonification or gross nitrogen mineralization.
The new paradigm regarding nitrogen cycling rethinks mineralization as the center point in the nitrogen cycle[11], and instead regards depolymerization of nitrogen containing polymers as what regulates the nitrogen cycle. Ammonification or gross nitrogen mineralization is the conversion of organic-nitrogen polymers to ammonium, mediated by heterotrophic microbes. The microbial degradation of amino acids, sugars, and nucleic acids is seen as a need for energy and carbon, with ammonium released as a byproduct of catabolism[9]. Since the polymers are not immediately bioavailable due to their large size, they are first cleaved by extracellular enzymes[11].
The extracellular enzymes are created by microorganisms, and have the capacity to depolymerize proteins, aminopolysaccharides found in cell walls, nucleic acids, and to hydrolyze urea[9]. Proteins are broken down by a wide variety of proteinases (large proteins), proteases, and peptidases that cleave dipeptides or split individual amino acids. Some examples of isolated proteolytic enzymes are subtilism, clostripain, and thermolysin[9].
Several common extracellular polymers degrade cell wall polymers, producing an end product of individual amino sugar monomers[9]. In fungi which contain primarily chitin cell walls, chitinase degrades the cell walls into dimers, and then chitobiase breaks down each dimer into N-acetylglucosamine[9]. In bacteria containing peptidoglycan cell walls, several enzymes, the most predominant of which is lysozyme, breaks the beta linkages holding the polymer together[9].
Nucleic acids are degraded by RNases and DNases, which hydrolyze ester ester bonds between phosphate and pentose sugars that make up the backbone of DNA and RNA, resulting in an end product of individual nucleotides[9].
Ureases hydrolyze urea into carbon dioxide and ammonium, which plays a key role in immediate fertilization plant availability[9]. The cofactor of the urease enzyme is nickel[9].
The research on extracellular enzymes and soil constituents has revealed some insight into the complexity of the soil matrix. An enzyme and substrate may bind to clay surfaces, protecting them from degradation and resulting in a slow degradation of the substrate over time[9]. In addition, an enzyme can remain active and act on an available substrate if its catalytic site is unaffected and structure unaltered by adsorption to the clay surface[9].
Intracellular enzymes play a critical role in nitrogen transformations[9]. In most cases, the final product of ammonium occurs as a result of intracellular enzymes within microbial cells. There are two types of nitrogen found in amino acids: amine and amide. In the amide, the asparagine and glutamate are cleaved by aspariginase and glutaminase. In the amine, amino acid dehydrogenases and oxidases release the amino nitrogen in a process called deamination.
Amino sugars are metabolized in two steps - kinase phosphorylation followed by deamination[9]. The degradation of nucleotides and subsequent ammonium release involves multiple steps - nucleotides are hydrolyzed to produce nucleosides and phosphate (PO43-), the nucleosides are then further hydrolyzed to purines and pyrimidine bases and pentose sugar molecule parts, and finally the ammonium is released during the catabolism of the purines and pyrimidines with a urea intermediate[9].
Key microorganisms
The groups of microorganisms responsible for ammonification are very broad and cover a wide range of species.
Organoheterotrophs are an example of a group of microorganisms that mineralize nitrogen, often going after carbon in addition to the nitrogenous compounds present in the plant residue.
Nitrogen Immobilization
Immobilization is the conversion of ammonium and nitrate to organic nitrogen, primarily as a result of the assimilation of ammonium by the microbial biomass.The process requires energy for the conversion of nitrate to ammonium and subsequent incorporation of ammonium into amino acids.
Microorganisms assimilate ammonium by two secondary pathways - the end result of both pathways is glutamate, which is then transferred to other carbon skeletons by transaminase reactions that form amino acids[9].
The first pathway occurs under a high concentration of ammonium (about 0.5 milligrams of nitrogen per kilogram of soil), in which NADPH2 coenzymes adds ammonium to alpha ketoglutarate in order to form glutamate[9].
The second pathway occurs in lower concentrations of ammonium and uses a glutamine synthetase-glutamate synthetase (GOGAT) pathway in which ATP is used to add ammonium to glutamate to form glutamine, and in combination with alpha ketoglutarate, to subsequently remove the ammonium to form two glutamate molecules[9].
The Equilibrium Between Ammonification and Immobilization
Ammonification and nitrogen immobilization are co-occuring processes, with ammonification occurring in the presence of a C/N ratio of less than 25[9].There are several factors that influence whether there is net production or consumption of nitrogen by microorganisms in soil, including factors influencing chemical pathway rates, substrate availability and composition, and the chemical composition of the soil matrix[9].
A very broad rule of thumb that estimates the direction of the equilibrium between ammonification and immobilization is the carbon to nitrogen ratio of the substrate. Various factors other than the C/N ratio may alter the equilibrium, such as lignin content, the moisture of the material being broken down, and the pH of the substrate and surrounding soil matrix[9].
These factors also alter the decomposition rate of the materials. However another broad rule of thumb is that the less carbon there is, the more quickly that the material will decompose and the nitrogenous compounds be made available.
The table below presents a few examples of C/N ratios[4].
| Material | C/N Ratio |
|---|---|
| Wheat Straw | 80:1 |
| Corn Stover | 57:1 |
| Flowering Rye Cover Crop | 37:1 |
| Vegetative Rye Cover Crop | 26:1 |
| Mature Alfalfa Hay | 25:1 |
| Ideal Microbial Diet | 24:1 |
| Rotted Barnyard Manure | 20:1 |
| Legume Hay | 17:1 |
| Hairy Vetch Cover Crop | 11:1 |
| Young Alfalfa Hay | 13:1 |
| Soil Microbes (Average) | 8:1 |
At C/N ratios above the ideal microbial diet (24:1) immobilization occurs due to the fact that there is a much greater amount of carbon that needs to be broken down – a process which requires more microbial biomass. Since the microbial biomass cannot retrieve the nitrogenous compounds until the carbon is broken down, the microorganisms convert inorganic nitrogen to organic nitrogen as an investment for their own growth.
Once the material is decomposed and there is an excess of microbial biomass, the microbial population begins to die off and return to equilibrium. The dead biomass, with a C/N ratio of 8:1, can be quickly utilized by the remaining biomass as a food and energy source or as a mineralization source for plant symbiosis.
C/N ratios below the ideal microbial diet cause mineralization due to the fact that there is an excess of organic nitrogenous compounds as a result of rapid substrate decomposition. The microbial biomass is not limited in growth by nitrogenous needs and organic nitrogen is converted to inorganic nitrogen for utilization for the purposes of plant growth and symbiosis.
Most organic matter is 45% carbon by mass, and thus the C/N ratio is determined mainly by the nitrogen concentration found in the residues[9]. In agricultural systems, it is critical to properly determine and control the nitrogen concentration in the applied crop residue in order to influence the equilibrium in a manner that is conducive to the goals of the agricultural system.
The net result of the equilibrium is influenced by biotic factors as well - 30% of yearly net mineralization is directly released by grazing and activities of soil animals such as protozoa and nematodes[9]. The same factors that influence the equilibrium in relation to microorganisms – soil moisture, pH, and the entirety of the soil matrix also have large roles in determining the activity of higher biotic agents and how much nitrogen they mineralize.
Key microorganisms
Heterotrophic microbes and other organisms assimilate ammonium to build up biomass. As in ammonification, microorganisms responsible for immobilization encompass a very broad group due to universal need for nitrogen for life.
Nitrification
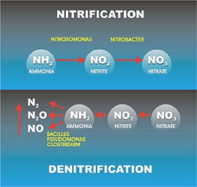
Nitrification is a two-step process of ammonium (NH4) to nitrate (NO3) by soil bacteria[11]. Ammonium is initially oxidized to nitrite by chemoautotrophs in the following manner[11]: NH3 + O2 --> NO2- + H+ + H2O
Chemoautotrophs are able to use carbon dioxide as a carbon source for the oxidation of ammonium. The most common bacteria that oxidizes ammonium is Nitrosomonas[11]. The second step of the nitrification process is the conversion of nitrite to nitrate: NO2- + O2 --> 2NO3- + 2H+ + 2e-
The most common group of bacteria that convert nitrite to nitrate are in the genus Nitrobacter. This group of soil bacteria obtain their energy from the ammonium oxidation process. Nitrite can be toxic to certain plants, thus the incomplete conversion of nitrite to nitrate may prevent plant growth in those areas[12]. It is important to ensure that nitrification occurs efficiently in food production systems as to prevent crop damage[12].
Denitrification
Denitrification is important in the continuation of the nitrogen cycle, as it is the step where gaseous dinitrogen is released back into the atmosphere. The natural cycle of denitrification comprises a series of enzymes such as bacterial NORs enzymes reduce nitrate to dinitrogen[13]. As a result, reduction of nitrates to gaseous dinitrogen follows a series of respiratory biochemical reactions by microorganisms[13]. Bacteria involved in denitrification are facultative anaerobic organoheterotrophs such as Bacillus[13]. The overall process of denitrification is as follows:
NO3- ------ > NO2- ------> N2O ---------> NO-------> N2
Nitrate -----> Nitrite ----> Nitric Oxide ----> Nitrous Oxide ------> Dinitrogen gas
Environmental Impacts

The nitrogen cycle causes many environmental issues such as global warming and pollution itself. The global increase in temperature is mainly due to the release of greenhouse gases. Greenhouse gases causes the blanket of air surrounding the earth to thicken, thus warming up the earth. The byproducts of the nitrogen cycle produce greenhouse effects, specifically in agricultural fields. Farmers input high amounts of ammonium nitrate to fertilize their soil to grow their crops. Consequently this increases the production of pollutants: nitrous oxide (N2O) and nitric oxide (NO), through denitrification[9]. CO2, another greenhouse gas, would also be produced from aerobic respiring microbes.
Excess nitrogen fixation has caused a lot of pollution into the biosphere. For example, nitrogen dioxide is degraded through photolysis to form oxygen radicals, which will react with molecular oxygen to form ozone in the troposphere, as shown in Figure 6. While ozone is beneficial in the stratosphere, where it can block UV radiation, ozone in the troposphere is a pollutant and can cause oxidative stress to humans and other organisms[3].
Mineralized nitrogen compounds in soil can be nitrified into nitrates, which can be leached into groundwater and cause contamination. Nitrate is soluble in water due to its negative charge; this polarization prevents adsorption onto the negatively charged soil surface and allows the soluble nitrate to leach into groundwater[3]. Nitrate pollution is a major concern because of its many adverse effects such as methemoglobinemia, cancer, and eutrophication. Nitrification not only produces nitrates but also produces nitric acid, which will acidify bodies of water and harm sensitive marine life[3]. Excessive nitrogen has also negatively affected terrestrial life by increasing the prevalence of pathogens, such as bacteria, viruses, and fungi. These pathogens were once limited by the lack of nitrogen but now have become more abundant[3].
Strategies to Lessen Environmental Impact
Some strategies to reduce mobile nitrogen in the biosphere are bioremediation, reduction of emissions, and sequestration. Bioremediation can reduce the amount of nitrate from the environment by converting it into atmospheric nitrogen and nitric oxide[9]. However, this has its own concerns because nitric oxide production must be limited to lessen effects of global warming. Reduction of emissions reduces the amount of nitrogen entering the biosphere, thus lessening the amount of mobile nitrogen available. Sequestration of nitrogen prevents nitrogen from becoming mobile and can be increased by selecting for crops with higher nitrogen uptake efficiency that are also able to form symbiotic relationships with nitrogen sequestering soil microorganisms[9]. There has been renewed interest in studying these relationships in the attempt to increase nitrogen sequestration in soil.
Current Research
Although the importance of soil microbes in the nitrogen cycle is well understood, current research is focused on the particular niches that different organisms occupy. Many of these experiments have identified some distinctive roles originally unknown to scientists, and are being studied for possible applications in agriculture and bioremediation practices.
One study highlights a symbiotic relationship between plants and a known predator that solidifies the networks within the nitrogen cycle. It was found that nematodes present in the soil consumed bacteria that did not produce the plant growth hormone indole acetic acid (IAA)[14]. As a result, there was less competition and increased resources for the IAA-producing bacteria, allowing them to proliferate under sufficient ammonia concentrations and produce more IAA for plant use. The increased availability of IAA to the plants allowed for increased growth rates. The study clarifies the roles of the different soil organisms and how they interact in relation to soil nitrogen levels, showing that indirect biological control of plant growth by the various soil food web organisms is a key feature in the nitrogen cycle[14].
Arbuscular mycorrhizal (AM) fungi benefically obtain nitrogen for their host plant[15]. Researchers measured this capacity for nitrogen sequestration as a potential method to reduce harmful nitrate leaching into soil and groundwater.Their experiment observed two types of host plants: Lolium, an obligate ryegrass with an affinity for AM fungi, and Trifolium, a legume with no preference for AM fungi. The amount of nitrogen in Trifolium’s biomass inoculated with AM fungi was significantly higher than its non-inoculated comparison. However, the Lolium’s nitrogen content was similar to the inoculated Trifolium. This signifies the critical relationship between host plants to their AM fungi in sequestering nitrogen. Researchers found consistent reduction in leached nitrate in both the grass and the legume. Compared to the control, the addition of AM fungi reduced the overall concentration of nitrate in both treatment soils. Lolium legume has a significant reduction in nitrate compared to the control, meaning that the AM fungi supplemented the processes of the nitrogen-fixing bacteria[15].
References
- ↑ Food and Agricultural Organization of the United Nation. 2003. Food Energy-Methods of Analysis and Conversion Factors. Rome. 2003. ISSN: 0254-4725. p. 7. URL: ftp://ftp.fao.org/docrep/fao/006/y5022e/y5022e00.pdf
- ↑ Malkki, S. 1999. Human faeces as a resource in agriculture. : 36-43. URL: http://orgprints.org/8477/
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., ... & Sutton, M. A. (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science, 320(5878), 889-892. URL: http://science.sciencemag.org/content/320/5878/889
- ↑ 4.0 4.1 USDA NRCS East National Technology Support Center, & North Dakota NRCS. (2011, January). Carbon to Nitrogen Ratios in Cropping Systems (United States, United States Department of Agriculture, Natural Resources Conservation Service). Retrieved March 11, 2016, from soils.usda.gov/sqi
- ↑ 5.0 5.1 5.2 Graham, P. H., & Vance, C. P. (2000). Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Research, 65(2), 93-106. URL: http://www.sciencedirect.com/science/article/pii/S0378429099000805
- ↑ 6.0 6.1 Zehr, J. P., Jenkins, B. D., Short, S. M., & Steward, G. F. (2003). Nitrogenase gene diversity and microbial community structure: a cross‐system comparison. Environmental microbiology, 5(7), 539-554. URL: http://onlinelibrary.wiley.com/doi/10.1046/j.1462-2920.2003.00451.x/full
- ↑ 7.0 7.1 Hoffman, B. M., Lukoyanov, D., Yang, Z. Y., Dean, D. R., & Seefeldt, L. C. (2014). Mechanism of nitrogen fixation by nitrogenase: the next stage.Chemical reviews, 114(8), 4041-4062. URL: http://pubs.acs.org/doi/abs/10.1021/cr400641x
- ↑ 8.0 8.1 8.2 8.3 The Microbial World, produced by Jim Deacon. Institute of Cell and Molecular Biology, The University of Edinburgh. Cited at 2016. URL:http://archive.bio.ed.ac.uk/jdeacon/microbes/nitrogen.htm
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 9.15 9.16 9.17 9.18 9.19 9.20 9.21 9.22 9.23 9.24 9.25 9.26 Sylvia, D.M., Fuhrmann, J.J., Hartel, P.G.,& Zuberer, D.A. (2005). Principles and application of soil microbiology. 2nd edition.Pearson. New Jersey.
- ↑ Fun Facts About Fungi, hosted by the Utah State University Intermountain Herbarium. Utah State University. Cited at 2016. URL: http://herbarium.usu.edu/fungi/funfacts/lichens.htm
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Schimel, J. P., & Bennett, J. (2004). Nitrogen mineralization: challenges of a changing paradigm. Ecology, 85(3), 591-602. URL: http://onlinelibrary.wiley.com/doi/10.1890/03-8002/abstract
- ↑ 12.0 12.1 Groffman, P. M., Altabet, M. A., Böhlke, J. K., Butterbach-Bahl, K., David, M. B., Firestone, M. K., ... & Voytek, M. A. (2006). Methods for measuring denitrification: diverse approaches to a difficult problem. Ecological Applications, 16(6), 2091-2122. URL: http://onlinelibrary.wiley.com/doi/10.1890/1051-0761(2006)016%5b2091:MFMDDA%5d2.0.CO%3b2/abstract
- ↑ 13.0 13.1 13.2 Gerardi, M. H. Introduction to Nitrification. Nitrification and Denitrification, 35-41. URL: http://onlinelibrary.wiley.com/doi/10.1002/0471216682.ch6/summary
- ↑ 14.0 14.1 Cheng, Y., Jiang, Y., Wu, Y., Valentine, T. A., & Li, H. (2016). Soil Nitrogen Status Modifies Rice Root Response to Nematode-Bacteria Interactions in the Rhizosphere. PLOS ONE PLoS ONE, 11(2). Retrieved March 09, 2016. http://www.be-md.ncbi.nlm.nih.gov/pmc/articles/PMC4739600/
- ↑ 15.0 15.1 Köhl, L., & Heijden, M. G. (2016). Arbuscular mycorrhizal fungal species differ in their effect on nutrient leaching. Soil Biology and Biochemistry, 94, 191-199. Retrieved March 10, 2016. http://www.sciencedirect.com/science/article/pii/S0038071715004125
Edited by student of Kate Scow