Wetlands
Introduction
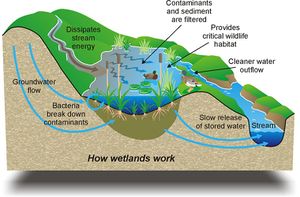
Wetland ecosystems are extraordinarily useful communities (National Resource Counsel 1992). They perform vital environmental functions (denitrification, water purification, flood control, etc) and provide more services per hectare than any other ecosystem (Craig et al. 2008, Richardson 2008). Along with these natural benefits, wetlands also have the ability to reduce the effects of anthropogenic pollution, such as wastewater treatment and excessive fertilizer removal (Keeny 1973, Lee et al. 1969, Nichols 1983). One of the most important functions that wetlands perform is their role in the transformation of nitrogen. Fertilizers generate high nitrate loads and wetlands have the ability to transform this into less harmful forms of nitrogen.
Denitrification is an especially important function carried out by wetland communities (Smith and Ogram 2008, Forshay and Stanley 2005, Craig et al. 2008) as excessive nitrate in the water can contribute to eutrophication. Left unchecked, eutrophication can lead to extensive algal blooms, hypoxia following decomposition of algal biomass, and an abrupt change in the makeup of the overall ecosystem. This phenomenon has been observed in both the Gulf of Mexico and Chesapeake Bay, and is mostly caused by the excessive amounts of fertilizer that end up in the waterways from extensive farming (Hey, 2002) along the Mississippi and Potomac rivers respectively (Galeone et al. 2006, Howarth et al. 1996, Malakoff 1998). Natural wetlands remove nitrate from the water and can be used to alleviate eutrophication. However, because of extensive habitat loss, nitrification of waterways increased drastically during the 20th century (Malakoff 1998, Walter and Merritts 2008).
Wetlands are vital communities, and provide a multitude of services to ecosystem function. They are incredibly diverse ecosystems and have large roles in primary production and floodwater retention. Perhaps one of the most important functions of a wetland is the habitats ability to purify water. Wetlands have the ability to aid in pollutant removal, and microorganisms present in the saturated soils of these wetlands play a large role in performing that function.
Main classes of Wetlands
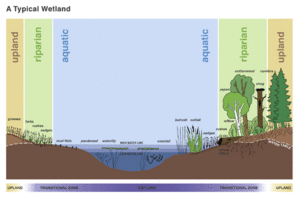
While wetlands can be found in a variety of regional and topographical locations, there are two general categories of wetlands recognized: coastal/tidal wetlands and inland/non-tidal wetlands.
Coastal Wetlands
These ecosystems are closely linked with estuary and salt marsh systems in that fresh water and salt water combine to form a wide array of salinities. In this environment, the constantly fluctuating water levels (from tidal action) and salt concentrations combine to form a difficult habitat. Certain plants have adapted to these variable conditions to form unique communities capable of flourishing in the extreme environment. These include mangroves, certain grasses, and other salt-tolerant trees and shrubs.
Inland Wetlands
Unlike coastal wetlands, salinity is not as big a contributing factor for inland wetland systems. While salinity is important for various plant and microbial communities, wild fluctuations in the salt concentration are not seen as frequently as in estuarine habitats. Inland wetlands are most common on floodplains along rivers and streams (riparian wetlands), but can also be found in land depressions, surrounding lakes and ponds, and anywhere else where the soil environment is under constant, or near constant, saturation (vernal pools and bogs) (USEPA). Riparian wetlands are unique because they allow the water to percolate through the system slowly as opposed to rushing down a stream channel. Because the water is spread out over a large surface floodplain, the hydric soil microbial communities, along with the plants present are able to filter out nutrients and other pollutants to help purify the water. Because inland wetlands cover a wide range of environmental conditions, classification is broken down further into types of wetlands based on region.
Physical Environment
Wetlands are classified as a transition between aquatic and terrestrial environments (Casey, 2001). Water hydrology (wetlands are usually saturated) generally determines the structure of the soil environment and the types of plant, animal, and microbial communities can inhabit the ecosystem. Because of the continual presence of water, conditions are created that support the growth of specially adapted plants and the formation of characteristic wetland soil – hydric soils. Wetlands are unique in that they actively support both aquatic and terrestrial species throughout the year (USEPA).
Hydrology
Water availability plays a huge role in determining the processes that can be performed by a wetland. In general, more saturated environments (aquatic wetlands and flooded riparian wetlands) experience higher rates of anaerobic respiration - like dentrification, methanogenesis, iron reduction, and sulfate reduction, and depressed rates of aerobic processes - like nitrification. Constant saturation causes oxygen to be depleted quickly, causing microorganisms to turn to other substrates for energy (Balser, 2006). Microorganisms are quite adept at using other available substrates for energy. Environments that experience wetting and drying cycles tend to be able to perform both aerobic and anaerobic functions depending on the conditions experienced. During wet cycles, anaerobic pathways can be used for energy (dentrification, etc) while in dry cycles, oxygen is present allowing for aerobic cycles to present themselves again.
Soil Structure
The layout of wetland soil plays a significant role in the processes performed by the community. The main identifying feature of a wetland is the presence of hydric soils – basically soils that function in strict anaerobic conditions under increased redox potential (USDA, 2004). In riparian wetlands, topsoil is generally found sitting on the surface, and is capable of performing aerobic functions because of the proximity to oxygen. Below the water line lie the hydric soils, gravel, and bedrock as you descend. The main factor influencing the structure and formation of hydric soils is the hydrology of the ecosystem. Communities that are constantly flooded (ie aquatic and some riparian wetlands) have constantly saturated hydric soils. These soils also act like sponges, helping alleviate flooding potential. The structure of the soil allows water to percolate through slowly, so when increased volume is added to the system, the soil itself can absorb some of the floodwater, mitigating some of the problems.
Biological interactions
Plants
Wetlands are characterized by a wide variety of plants that can inhabit the saturated environment. The most common of these are cattails, bulrushes, sedges, water lilies (known as emergent vegetation) and pondweed and waterweed (known as submergent vegetation). These plants play a vital role in ecosystem function in that they help in various biogeochemical cycles. As the most productive ecosystem on earth, wetlands provide an enormous amount of dissolved organic matter through the process of photosynthesis and subsequent death and decomposition. The wide variety of plant life and subsequent pool of dissolved organic matter is vital in creating vibrant wetland communities and accounts for the wide diversity of organisms seen in marsh environments. Plants are not the only organisms capable of photosynthesis. Wetland communities have large populations of cyanobacteria and algae – capable of also fixing carbon dioxide into a useful substrate. This is important because it provides the foundation of the extensive food web found in wetland communities.
Animals
A variety of insect and animal species can inhabit wetland environments. The availability of standing water makes the habitat an ideal breeding ground for a host of insect species including mosquitoes and gnats. The overabundance of algae and photosynthetic bacteria also provides the insect populations with an easy source of food. Wetlands are particularly important habitats for amphibians and reptiles because of the proximity of open water to vegetated areas. Also, because of the wide array of insects inhabiting the ecosystem, a plentiful source of food is available for the amphibians and reptiles. Larger mammals and birds also are plentiful in marshy environments, again because of the abundance of food found. Overall, the food web found in wetland conditions is often the most complex and involved simply because of the abundance and diversity of life found in the area.
Microorganisms
Microorganisms play vital roles in the food web, functioning as primary producers and decomposers. Some microorganisms are primary producers – photoautotrophic organisms who glean energy from light. These are fundamental in ensuring the strong food web observed because they provide the essential energy needed to higher trophic levels. When these higher trophic organisms die, microbes decompose the plant or animal to gain back valuable energy and reintroduce it into the system as dissolved organic carbon. This overall process is known as the microbial loop.
Microbial processes
Wetlands microbes mediate many of the vital biogeochemical processes needed in the environment. The carbon, nitrogen, phosphorus, sulfur, and iron cycles all have some role in wetland communities and the bacteria present in the anoxic hydric soils are often responsible for the various oxidations and reductions that occur.
Carbon Cycle
Microbes are very important in the carbon cycle. Many photoautotrophs are responsible for the initial fixing of carbon dioxide into useful sugars that can be used for energy. Aside from primary production, decomposition is also a function of microbial communities in wetland soils. Because of anaerobic conditions, decomposition rates are slow, but overall soil organic matter (SOM) is quite high. Microbial communities in hypoxic conditions have the ability to transform this organic matter into usable forms of mineralized dissolved organic carbon. This process allows plants and other organisms to use these substrates once again for energy. If mineralization did not occur, then carbon would stay in an organic form and be unusable to plants. Microbial communities in the soil can mineralize the SOM into inorganic forms of carbon, like carbon dioxide, that plants can then use for photosynthesis once again.
Under extremely reduced conditions, where no good terminal electron accepters are available, microbes can use carbon dioxide. These methanogenic bacteria use the CO2 as a TEA resulting in the production of methane (CH4) also known as swamp gas. Another group of bacteria, known as methanotrophs, use the methane as their energy source and oxidize it to CO2. In general, methanotrophs are obligate aerobes, meaning that in hydric soils, they will be active right above the aerobic/anaerobic dividing line. Methane is a major greenhouse gas, but because of the placement of methanotrophs, up to 90% CH4 generated in hydric soils can be consumed before it reaches the atmosphere (USDA, 2004).
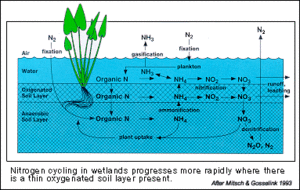
Nitrogen Cycle
The nitrogen cycle is perhaps the cycle that feels the greatest influence from microbial activities. One of the most importance processes carried out by soil microbes is bacterial denitrification – the process of converting nitrate (NO3-) to gaseous nitrogen compounds (N2, N2O, NO). This process is used by facultative anaerobic bacteria as a means to use nitrate a terminal electron acceptor (TEA). Normally, the most energetically favorable TEA is oxygen, but because hydric soils operate in hypoxic conditions, microbes must turn to other compounds to complete the phosphorylation pathway. This process is the primary removal mechanism of dissolved N in wetland communities. This is an extremely important process because of the excessive amounts of fertilizers used for agricultural purposes. Without denitrifying populations of bacteria, the excess nitrate would remain in the aquatic system causing an explosive growth of algae. Ultimately this process would lead to the creation of a dead zone and cause extensive ecological and economic damage.
A similar process to this is dissimilatory nitrate reduction in which bacteria convert nitrate all the way to ammonium, which is then released by the cell. This process is favored by a high ratio of available C to NO3-. This is because the microbes need useable forms of nitrogen, and the conversion all the way to ammonium creates and inorganic form of nitrogen usable to both microbes and plants. Also, a select few groups of chemoautotrophic bacteria can get energy from oxidizing ammonia to nitrite (NO2-) and subsequently nitrate.
Other organisms are capable of nitrification (the process of converting N2</sub?> to ammonia), but this process is not as prevalent a pathway as denitrification. Nitrification requires an extensive energy input to convert nitrogen gas to ammonia, and the process is usually only done under conditions of low nitrogen availability. In general, wetlands have high concentrations of available nitrogen (in the form of NO3- and NH3), so the nitrification pathway is not readily used.
Iron and Manganese
When nitrate and oxygen are not readily available as TEA’s, microbes must turn to other oxidized compounds in an effort to gain energy. Both Fe3+ and Mn4+ have the ability to be reduced by bacteria and fungi under strict anaerobic conditions as TEA’s, resulting in the formation of Fe3+ and Mn3+. While they will not yield as much energy for the organism, it will still allow anaerobic respiration to continue. However, this process is controlled largely by oxygen availability and redox conditions. When oxygen is present, that will be used as the TEA and chemoautotrophic bacteria will oxidize the reduced forms of iron and manganese back to the original +3 and +4 oxidation states respectively.
Sulfur
Another possible compound that can be used by bacteria as a TEA is sulfate (SO42-). In the reduction process, sulfate is converted to either elemental sulfur or hydrogen sulfide (H2S), which gives off the characteristic smell of rotting eggs. Sulfur-oxidizing bacteria, on the other hand, have the ability to oxidize the sulfides and elemental sulfer back to sulfate, or some other partially oxidized form of sulfur. While this is a useful process, bacteria often will use any available oxidized substrate before sulfate as a TEA. The reduction of sulfate will give the organism energy, but it will be nowhere near the amount gained as if the organism had used oxygen, nitrate, iron, or manganese.
Key Microorganisms
Bacteria
Bacteria are present in high diversity in wetland environments. The largest group of wetland bacteria is proteobacteria – capable of a number of important functions ranging from nitrogen fixation, to denitrification, to iron and sulfate reducers. These are chemotrophs – gaining their energy from chemical sources as opposed to light (or photosynthetic) energy. Other chemotrophic bacteria are actinomycetes and firmicutes. Both of these are found in lower abundance in wetland communities due to low decomposition rates, but they are present in small amounts. Some examples include:
- Proteobacteria
- β-Proteobacteria
- Nitrospira (nitrate reductions – denitrification)
- Nitrosomonas (ammonia oxidations)
- γ-Proteobacteria
- Pseudomonas (capable of degrading contaminants – naphthalene, toluene, etc.)
- δ-Proteobacteria
- Desulfovibrio (sulfate reducers)
- Geobactor (Iron reducers)
- β-Proteobacteria
- Actinomycetes – resemble fungi with filamentous growth form
- Streptomyces – most common actinomycetes group (degrade resistant substrates)
- Arthrobacter (degrade toxic compounds)
- Firmicutes
- Bacillus (falcultative aerobes)
- Clostridium (anaerobic bacteria capable of using various TEAs)
There are also photosynthetic bacteria present in wetlands. The primary photosynthetic bacteria group is cyanobacteria. Often time, these will form symbiotic relationships with plants, because of their capability to fix nitrogen into a useful inorganic form (ammonium).
Archaea
Archaea are the organisms responsible for the sulfate reductions that occur in wetlands, along with a good portion of the ammonia reductions. These lithotrophic organisms are almost exclusively anaerobic in wetland environments and are classified as nitrifiers, methanogens, and anaerobic methane oxidizers. Some of the common organisms found in this domain include:
- Euryarchaeota
- Methanobacteria (methanogenesis)
- Methanosarcina
- Crenarchaeota
- Euryarchaeota
Eukaryotes
Algae, classified as eukaryotes, also undergo photosynthesis to obtain energy and are a primary source of food for higher trophic levels. Other higher organisms, like plankton, daphnia, and ciliates are also integral parts of wetland communities, but are generally higher up in the trophic level, making them heterotrophs, and thus reliant on lower trophic levels for energy. As far as wetland function goes, bacteria and archaea are the primary drivers in biogeochemical cycling.
One eukaryotic organism that is relatively important to nutrient cycling is fungi. Normally an important decomposer, fungi are present in relatively low amounts in wetland communities because of the constant saturation and anoxic conditions. Because of the anoxic conditions, decomposition rates are low, limiting the importance of fungi in the environment.
Wetland Loss
Land changes, mostly brought about by human industrialization, have significantly reduced the acreage of this vital habitat, as wetlands were once considered useless features of the landscape (Vitousek et al. 1997). However, this view has been reversed, and land developers have recognized the importance of having these ecosystems around. In the United States, the government instituted a “no net loss” policy, dictating that the total acreage of wetlands must not decrease any further. This law gained new importance after the tragic loss of life in the New Orleans area after hurricane Katrina. In February of 2005, a report was published by National Geographic documenting how devastating a hurricane could be to the region because of the significant loss of wetlands in the region (Handwerk 2005). These lost wetlands could have significantly reduced the storm surge and prevented the loss of hundreds of lives (Handwerk 2005). Because wetland soils are porous, water from floods or storm surges are effectively dampened when they pass through the marshy terrain (Middleton 1999). By recreating these habitats along rivers, spring flood damage can be lessened by the buffering effect of wetlands.
There are some factors working in favor of recreating proper ecosystem function. In many cases, wetland soils were buried during land use changes and not completely uprooted or destroyed. Because microbes are so resilient, it is possible that once these remnant wetland soils are uncovered and restored, the microbes that have lain dormant for decades can return to normal function if appropriate environmental conditions are established (Orr et al. 2007).
Current Research
Monitoring denitrification rates at restored wetlands
One large area of ongoing research has focused on individual wetland restoration/mitigation projects, usually at the site of a former or currently degraded wetland. While many of these projects have been successful at producing a wetland, they have often focused on restoring the floodplain and macro-ecology rather than the microbial ecology necessary for biogeochemical cycling (Orr et al. 2007, Richardson 2008). Even wetlands that are classified as “successful” may fail to deliver microbially-mediated ecosystem services like denitrification. In the Orr et al. paper (2007), a floodplain was reconnected to the Baraboo River system by removing a series of levees. The area was restored and it was expected that the reconnected floodplain would allow for rapid denitrification of the river. Following restoration, however, it was found that while the potential for denitrification was present, the improved floodplain did not noticeably improve denitrification rates (Orr et al. 2007). Even though the macro-ecology was accurately reproduced, the restoration effort did not achieve its overall goal of significantly enhancing denitrification rates.
Temporal microbial community shift during wetlands restoration
Because of the role played by microbial communities during biogeochemical cycling, a huge effort has been made to ensure that microbial community composition of restored wetlands mimics that of natural, unharmed wetlands (Bossio, 2006; Peralta et al., 2007). This can be done in one of two ways. The first method often used is high throughput, genotypic techniques. In general, these methods attempt to determine if the structure of the restored wetland appears similar to that of the natural wetland. Lab procedures like BIOLOG assays, PLFAs, PCR techniques, and others determine if the function of the two communities are similar. Using genes, substrate utilization, or other indicators, it can be determined if the two communities, even if phylogenetically different, have the ability to do the same function (denitrification, nitrification, etc.) The second method involves culturing the microbes found on site in an effort to determine phylogenetically what inhabits a given site. The problem with this method is that less than 1% of bacteria are able to be cultured. So while this technique may give some phylogenetic data, the overall diversity is grossly underestimated. These techniques allow for monitoring of the community over time to see if the restoration has any affect on the makeup of the microorganisms inhabiting the soil.
Wetlands as waste treatment plants
Water purification is an important function of wetland ecosystems. As mentioned above, microbes have the ability to remove excessive amounts of nutrient runoff from agricultural/human sources. One big area of recent research has been the area of wastewater treatment. The extensive diversity of plant, animal, and microbial life allows wetlands to remove pollutants and purify water at an extremely high rate (USEPA, 1993). It has been repeatedly observed that suspended solids and oxidized nutrients are readily used by wetland organisms. As the water percolates through the system, these substrates are removed from the aquatic environment either through adsorption to the soil (phosphates and large organic compounds), microbially mediated removal (biochemical reactions), or uptake into plants (heavy metals, and some organic compounds). The resulting output of water is substantially cleaner than the inflow, showing how effective wetlands can be at water purification.
Resources
Mid-Atlantic guide to hydric soils and microbial processes
Wetland Wastewater Treatment studies
References
Balser, T., K. McMahon, D. Bart, D. Bronson, D.R. Coyle, N. Craig, M. Flores-Mangual, K. Forshay, S. Jones, A. Kent, A. Shade. 2006. Bridging the gap between micro- and macro-scale perspectives on the role of microbial communities in global change ecology. Plant and Soil 289:59-70.
Bossio et al., 2006. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biology & Biochemistry 38 (2006) pp. 1223-1233.
Casey, R. E., Klaine, S. J., Nutrient Attenuation by a Riparian Wetland during Natural and Artificial Runoff Events. J. Environ. Qual. 30:1720–1731 (2001).
Craig, LS, MA Palmer, DC Richardson, S Filoso, ES Bernhardt, BP Bledsoe, MW Doyle, PM Groffman, BA Hassett, SS Kaushal, PM Mayer, SM Smith, and PR Wilcock. Stream restoration strategies for reducing river nitrogen loads. 2008. Frontiers in Ecology and the Environment 6:529-538.
Forshay KJ, Stanley EH. 2005. Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry 75: 43–64.
Galeone DG, Brightbill RA, Low DJ, O’Brien DL. 2006. Effects of streambank fencing of pastureland on benthic macroinvertebrates and the quality of surface water and shallow ground water in the Big Spring Run basin of Mill Creek watershed, Lancaster County, Pennsylvania, 1993-2001: Scientific Investigations Report 2006-5141, 183 p.
Handwerk, B.2005. Louisiana coast threatened by wetlands loss. National Geographic. Feb. 2005.
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T. 1996. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry 35: 75-139.
Keeny, D.R. 1973. The Nitrogen Cycle in Sediment-Water Systems. Journal Environ. Quality 2(1):15-29.
Lee, G, E., Bentley and R. Amundson. 1969. Effect of Marshes on Water Quality. University of Wisconsin, Madison.
Malakoff, D. 1998. Death by Suffocation in the Gulf of Mexico. Science 281:190-193.
Middleton, B. 1999. Wetland restoration: flood pulsing and disturbance dynamics. John Wiley and Sons, New York.
National Research Council. 1992. Restoration of aquatic ecosystems: science, technology, public policy. National Academy Press, Washington, D.C.
Nichols, D. 1983. Capacity of Natural Wetlands to Remove Nutrients from Wastewater. Jour. Of Water Poll. Control Fed. 55(5):495.
Orr et al., 2007. Effects of restoration and reflooding on soil denitrification in a leveed Midwestern floodplain. Ecological Applications 17(8), 2007, pp. 2365-2376.
Peralta, A.L., J.W. Matthews, D.N. Flanagan, and A.D. Kent. 2007. Microbial community structure and function in restored floodplain forest wetlands. Proceedings of the International Symposium on Soil Biodiversity and Ecology. Taipei, Taiwan.
Richardson CJ (2008) The Everglades Experiments: Lessons for Ecosystem Restoration (Springer, New York) p 698.
Smith, J. M., and A. Ogram. 2008. Genetic and functional variation in denitrifier populations along a short-term restoration chronosequence. Applied and Environmental Microbiology. 74(18):5615-5620.
Vitousek, P. M., H. A. Mooney, J. Lubchenco, and J. Melillo. 1997. Human domination of Earth’s ecosystems. Science 277:494–499.
Walter RC and Merritts DJ. 2008. Natural streams and the legacy of water-powered mills. Science 319:299-304