Zaire ebolavirus: Pathogenesis, History, and Treatment of the Deadly Virus
Introduction
By Alexander Manning
Ebola is an infectious virus that struck fear into countries all around the world in 2014-2015. This disease is capable of causing an incredibly painful and brutal death. The virus is capable of invading a human host and causing an incredibly vast array of complications[3]. The virus doesn't only disrupt the function of organs, but has developed ways of evading the immune system which lead to the virus being very difficult to detect and stop[3]. There are many different strains of the virus, all responsible for various outbreaks throughout the world, in particular Africa. The virus was discovered in Africa approximately 40 years ago in the Democratic Republic of the Congo, where it's deadly spread across the continent started[6]. This notorious virus was only discovered a few decades ago, but since its discovery, the virus has developed a reputation for being one of the deadliest viruses known to humankind.
History of the Virus
The Ebolavirus was first discovered in 1976 near the Ebola River in the Democratic Republic of the Congo [6]. In that year, there were 318 confirmed cases of the virus infecting humans, resulting in 280 deaths. Over the next several years, there were only a few reported cases, usually in controlled situations that didn't result in any fatalities. Then, in 1995, there was an outbreak of the virus in the Democratic Republic of the Congo. This outbreak resulted in 315 confirmed cases with 250 deaths reported. Just five years later, there was an outbreak in Uganda that resulted in 425 cases and 224 deaths. This outbreak was believed to have been a result of the attendance of a funeral. The person being buried was killed as a result of the Ebolavirus infection, and the people attending the funeral were not wearing the proper attire to prevent transmission of the virus. The following year, there was another outbreak, this time in the Republic of Congo. This outbreak saw 57 cases with 43 deaths. Six years later in 2007, there were two separate outbreaks. One outbreak was in western Uganda, the other in the Democratic Republic of the Congo. The Uganda outbreak had 149 cases with 37 deaths. The outbreak in the Democratic Republic of the Congo had 264 cases with 187 reported deaths. Seven years later, in 2014, the largest outbreak in history broke out in western Africa. During this devastating outbreak, there were over 27,000 reported cases with over 11,000 reported deaths[6]. This virus has now been wreaking havoc on the world and western Africa in particular, for four decades now. This virus has claimed thousands and thousands of human lives. This has terrified countries into preventing citizens from returning home on the chance that they could spread the virus causing an epidemic that would render their country helpless. Originally discovered a few dozen miles away from a river in the Democratic Republic of the Congo, this virus has since developed a stigma making it known for the brutal death it is capable of causing. Vast amounts of research have been done on the virus. All aspects of the virus have been considered, from function and pathogenesis to treatment and vaccinations. [6]
The Ebolavirus is seen as one of the most deadly viruses known to humans with a 50-90% mortality rate [2]. There are currently five known strains of the virus all depending on the location of the virus. However, only four of the five viruses are capable of causing infection in a human host. This fifth virus is capable of infecting primates [2].
Virus Entry and Pathogenesis
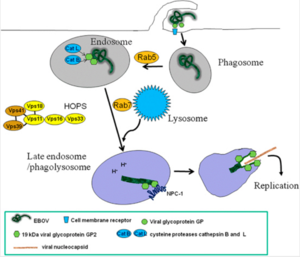
The Ebolavirus enters the host through basic entry paths. It can enter through mucous membranes, broken skin, or by parental transmission [4]. One way that the virus is so deadly is its ability to attack almost every type of human cell. It is capable of binding to these membranes of essentially every human cell except for lymphocytes. The entry of the cell is controlled by a glycoprotein that is responsible for binding the virus to the cell receptors. Once the virus is bound to the cell receptors, the membranes fuse together, followed by the opening of the capsid injecting the genome of the Ebola virus into the cell. The genome then undergoes transcription and replication. [4] In figure 2, the infection cycle of the virus is in image form. The figure shows the process that the virus goes through up until the replication of the viral genome.
Cytokines deregulation.
One of the major targets of the Ebolavirus is the cytokine system[3]
. The specific cytokines the virus provokes are the proteins that prompt the natural inflammatory response. However, Ebolavirus induces a cytokine dump, resulting in the overactive response of the inflammatory response. Essentially, the inflammatory response induced by the infection of the virus is so drastic that it begins to cause damage to the host cells. In studies of fatalities, there were levels of cytokines responsible for the inflammatory response that were drastically too high. The cytokine levels continued to rise until a few days before death occurred. As the virus continues to spread throughout the body, the immune system’s inflammatory response acts accordingly. In addition to the cytokine dump induced by the virus, the response ultimately does more damage to the host than good. Another result of the cytokine production is the attraction of leukocytes to the area. When all of these leukocytes are in close proximity, it can cause adhesion, rolling, and diapedesis of the leukocytes. This can lead to clinical hemorrhaging in the area. By deregulating the cytokines, the virus is able to spread rapidly throughout the host. [3]
Evading of the Type-1 INFs Response.
INFs response, or interferon response, is a major part of the immune system and its ability to fight off viral infections. In many patients infected with the Ebolavirus, there was a lack of type-1 INFs response [3]. This is due to the Ebolaviruss capability to inhibit the synthesis of proteins that activate the response when the cell has been infected. A protein produced by the Ebolavirus, VP35 is capable of inhibiting the synthesis of a specific INFs protein. It does this by disrupting multiple pathways in the synthesis process. Another way that the virus disrupts the INF response is through the production of protein VP24. This protein synthesized by the virus is particularly good at inhibiting the transcription of INFs response genes. A third way the virus is capable of defending itself from the antiviral response of the human body is through the glycoprotein inhibition of tetherin expression. Tetherin is responsible for the blocking of a budding virus to exit the cellular membrane of a host cell. With this mechanism blocked, the host cell has a much harder time controlling the virus from spreading between cells. Once the virus begins budding, the copies of the genome can easily be spread throughout the cell, especially when the Type-1 INFs Response has already been inhibited due to the above proteins. [3]
Attack of the liver and other essential organs.
The liver has been labeled as the “master chemist” of the human body, being responsible for synthesis of several proteins, chemicals, breaking down. With an improperly functioning liver, the body doesn't stand a chance against a virus. This is one of the reasons that Ebolavirus is very effective in the takeover of the host. When the virus spreads throughout the body, one of its targets are hepatic cells, or liver cells. The Ebolavirus is capable of inducing necrosis of the hepatic cells, which can result in multiple outcomes [3]. One study found that the induced necrosis of the hepatic cells helps explain the unusual hemorrhaging of the infected patients. When the virus is killing the hepatic cells, the liver can’t synthesize enough coagulant proteins, allowing the hemorrhaging to happen. The necrosis doesn’t only shut down the hemorrhaging proteins but also shuts down the liver functions, rendering the liver useless to the infected human. However, there are other animals and even primates that are capable of being infected with the virus, but don't express the same liver failure as humans. This has lead to the beginning of studies on these animals and primates to determine if there is a way to produce a similar response in humans that could lead to a more effective vaccine. Other organs that undergo a similar attack from the virus are the spleen, thymus, and lymph nodes. Similar to the liver, these organs see lymphatic depletion and necrosis due to the infection. [3]
Virus Structure
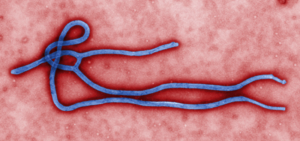
The genome of the Ebolavirus isn’t very complex, only containing enough genetic information to code seven to eight proteins. The virus is made of RNA, the glycoproteins, a membrane, and matrix proteins designed to help the virus keep its shape [4]. In figure 1, the entire virus is pictured via electron microscope. It has an abnormal shape consisting of long, tail-like sections as well as other sections that are twisted closely together.
The way the virus gets its genome into the host cell is via the glycoprotein [4]. The glycoprotein binds to receptors of the host cells. The protein extends from the virus and is covered in carbohydrate chains, which help the virus hide itself from the host’s immune system. The glycoprotein also functions as a viral fusion protein. The glycoprotein binds to the host cell where it changes shape. This change quickly pulls the virus and the host cell very close together, rapidly, fusing their membranes. This process is very similar to the glycoloprotein of an influenza strain and the glycoprotein of HIV [4].
Ebolavirus is a part of the Filoviridae family of viruses [3]. The size of the virus varies, depending on the strain. The Filoviridae family varies in size with a genome of negative-sense RNA. The length of the genome is approximately 19 kb long. In the RNA, there are seven open reading frames resulting in eight different proteins that have various jobs from matrix proteins to non-structural proteins. These eight proteins are responsible for the function of one of the deadliest viruses known to humans. [3]
The genome of the virus is protected by the nucleocapsid[4]. The purpose of the nucleocapsid is to protect the genome until the virus is attached to a host cell, so the genome can be injected into the host, where it can undergo replication and infect the cell. The nucleocapsid of the Ebolavirus is made up of nucleoproteins that surround the RNA in a helical fashion. The capsid proteins aren’t incredibly rigid, allowing the virus to have some flexibility. One of the proteins that makes up the capsid is very important not just for structure but also for replication of the genome. This protein is Polymerase (L) Protein. Once the genome is inserted into the host cell, this protein is responsible for the replication of the genome [4].
The matrix protein in Ebolavirus has been named the VP40 protein[4]. The matrix of the virus is responsible for the shape and drives the budding process of the host cell post infection. The protein is also responsible for being a connector between the nucleocapsid and the membrane of the virus, again helping the virus maintain a shape. Matrix proteins commonly have several jobs in the viral process. Ebola matrix protein has three distinct confirmations with three different functions in the cell. One confirmation is a hexamer responsible for priming the RNA to initiate replication once the virus is inside the host cell. The next confirmation is an octamer that is responsible for regulating the transcription of the genome. A final confirmation is a dimer that’s major function is protein transport. [4]
Host Response and Treatment
Current research has shown that the best outcome from Ebolavirusinfection is the body’s immune response in the form of antibodies[3]. Along with this suggestion, the research also explains that there is a drastic need for T cells in the response to fight the virus. The research was conducted on mice. When a mouse was injected with a lethal amount of the virus, serum and splenepocytes were both needed to help the mouse survive the virus. If only one of the two were injected into the mouse, then fatality ensued. This validates the need for both in the body’s response to the virus. This research also found that in patients that died from the virus; there was a drastic drop off in T cell production preceding death. In patients that survived the virus, there was a high rate of T cells. The Ebolavirus is capable of making the cells undergo apoptosis, effectively rendering the T cell response useless. In order to adequately produce T cells, the body must undergo three different processes: MHC-peptide must be recognized by TCR, stimulation of T cells and dendritic cells, and the proper environmental factors. If all of these necessary things occur, then the host’s immune system can properly produce T cells. This is essential for fighting off the virus. In this research, it was concluded that T cells are an important factor in controlling the initial infection. However, their larger purpose is controlling the virus past the initial infection, and plays a key role in helping eradicate the virus from the body. [3]
The Ebolavirus has varying incubation periods of three to twenty-one days[7]. The beginning symptoms of infection are similar to that of the flu but are followed by hemorrhaging, total organ failure, and symptoms representative of shock. Ever since the 2000 outbreak, the attempt to find the best way to treat and eliminate the virus has been a research point. Of the several attempts to treat this deadly virus, two outcomes have shown the most promise. The first is MB-003, an antibody cocktail that has human or a human-mouse hybrid group of antibodies to use against the virus. The study showed that MB-003 worked best when there were symptoms of the infection beginning to show, which could have been as soon as three days after infection. The second is ZMAb, another cocktail of antibodies used to attempt to defeat the virus post infection. The research found that ZMAb was most effective with countering the virus when the cocktail was taken within 72 hours after the host had been exposed to the virus. What makes these two treatments effective is their ability to assist the body’s natural response to the infection and assist the immune system with stopping the spread of the virus throughout the entire body. [7] As seen in figure 3, MB-003 had a higher rate of survival than the control group and the historical group. Historical control and control groups saw survival percentage drop to 25% and 0% respectively. Once the MB-003 recipients reached approximately 12 days post treatment, all subjects survived. Both control and historical control groups didn't see survival past day 11, post infection.
There is a new cocktail being synthesized currently, that is attempting to take the best aspects of both MB-003 and ZMAb and create a new therapeutic drug[8]. This new drug is called Zmapp. There were originally three different combinations of antibodies coding proteins from MB-003, and ZMAb to concoct Zmapp. Of the three cocktails produced, Zmapp 1 and Zmapp 2 were the most effective against the deadly virus. Since Zmapp 3 wasn’t as effective against the virus, the combination of antibodies and proteins was disregarded and the other two Zmapps continued to be researched in hopes of determining a therapeutic treatment for the virus. In the next round of testing, all animals that received Zmapp for treatment survived, while every animal in the control group died due to the infection from the virus. A potential problem with Zmapp is that there are four deadly versions of the virus, all of which have some differences with proteins functions, shape, etc., but maintain the same basic pathogenesis. So, a potential problem is Zmapp not being able to bind properly to the virus rendering the drug useless against that strain. The testing that found the drug effective was against the Kikwit strain of the virus. This wasn’t the strain responsible for the largest outbreak of the virus. The outbreak was caused by the Guinea strain of the virus, which ran rampant through western Africa for several months. So, an experiment was done to determine if Zmapp was going to be effective against a different strain. The results of the experiment showed that the Zmapp was still effective against the different strain, even though there were differences is molecular structures. This was an important discovery that could help lead to an even better synthesized product to combat the deadly virus. This product has moved towards being tested in human subjects, making FDA approval a likely outcome. If this product proves itself useful in combating the disease in humans, then Zmapp could have a drastic impact on future breakouts of the deadly virus. [8]
Ever since there was the incredibly large outbreak in western Africa, there has been a push to produce a vaccination for the disease. Originally, there were some attempts to incorporate a plasmid containing the proper protein synthesis genes into a host cell[7]. However, just recently in the year 2015, there was a vaccine synthesized that could effectively prevent the host from being infected even post exposure to the virus. This vaccine was named the rVSV-ZEBOV vaccine. The vaccine was originally synthesized by the Public Health Agency of Canada, before two pharmaceutical companies, Merck and New Link Genetics, further developed it. These two companies took the vaccine and refined it to produce a viable option for treatment. The rVSV vaccine is effective due to its capability to produce Ebolavirus glycoprotein as an immunogen. When the vaccine enters the body, it initiates an immune response. This response now recognizes the glycoprotein as the virus and builds the necessary antibodies against it. This allows for the body to defend itself against the virus if an infection happens after vaccination. However, the vaccination also works after exposure to the virus. Since the virus might not be potent enough directly after exposure, this allows the vaccine to take effect by “amping up” an immune response, effectively knocking out any viral molecules corresponding to the vaccination. However, the limitations of this vaccine are still being tested. Since the four different strains of the virus have differing the glycoprotein structures, the effectiveness of the vaccine might not be widespread and may only work on the original virus it was designed to prevent. [7]
Conclusion
The Ebolavirus is one of the most deadly viruses known to mankind. It has effectively wiped out large populations of people in Africa for a little over 40 years. The virus originally was affecting small groups of a few hundred people, but in 2014, the virus ran rampant in western Africa, killing over 11,000 people in just a few months. This was international news, causing vast panic in countries near the outbreak and even across oceans in the United States of America. Since it was discovered, research has been done on the virus to determine how the virus works, what proteins it produces, the pathogenesis, how many strains, and potential vaccinations and treatments. This small, simple virus, with only seven genes, will go down in history as one of the most notorious viruses to ever wreak havoc with the human race.
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11]
- ↑ Callaway, Ewen. "Successful Ebola Vaccine Provides 100% Protection in Trial." Nature (2015): n. pag. Web. 24 Apr. 2016.
- ↑ "Ebola Virus Disease (EVD) Information for Clinicians in U.S. Healthcare Settings." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 22 Feb. 2016. Web. 24 Apr. 2016.
- ↑ Falasca, L., C. Agrati, N. Petrosillo, A. Di Caro, M. R. Capobianchi, G. Ippolito, and M. Piacentini. "Molecular Mechanisms of Ebola Virus Pathogenesis: Focus on Cell Death." Cell Death Differ Cell Death and Differentiation 22.8 (2015): 1250-259. Web. 25 Apr. 2016.
- ↑ Goodsell, David. "Ebola Virus Proteins." Ebola Virus Proteins. PDB-101, Oct. 2014. Web. 24 Apr. 2016.
- ↑ Marzi, A., H. Ebihara, J. Callison, A. Groseth, K. J. Williams, T. W. Geisbert, and H. Feldmann. "Vesicular Stomatitis Virus-Based Ebola Vaccines With Improved Cross-Protective Efficacy." Journal of Infectious Diseases 204.Suppl 3 (2011): n. pag. Web. 24 Apr. 2016.
- ↑ "Outbreaks Chronology: Ebola Virus Disease." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 31 Mar. 2016. Web. 24 Apr. 2016.
- ↑ Pettitt, J., L. Zeitlin, D. H. Kim, C. Working, J. C. Johnson, O. Bohorov, B. Bratcher, E. Hiatt, S. D. Hume, A. K. Johnson, J. Morton, M. H. Pauly, K. J. Whaley, M. F. Ingram, A. Zovanyi, M. Heinrich, A. Piper, J. Zelko, and G. G. Olinger. "Therapeutic Intervention of Ebola Virus Infection in Rhesus Macaques with the MB-003 Monoclonal Antibody Cocktail." Science Translational Medicine 5.199 (2013): n. pag. Web. 24 Apr. 2016.
- ↑ Qiu, Xiangguo, Gary Wong, Jonathan Audet, Alexander Bello, Lisa Fernando, Judie B. Alimonti, Hugues Fausther-Bovendo, Haiyan Wei, Jenna Aviles, Ernie Hiatt, Ashley Johnson, Josh Morton, Kelsi Swope, Ognian Bohorov, Natasha Bohorova, Charles Goodman, Do Kim, Michael H. Pauly, Jesus Velasco, James Pettitt, Gene G. Olinger, Kevin Whaley, Bianli Xu, James E. Strong, Larry Zeitlin, and Gary P. Kobinger. "Reversion of Advanced Ebola Virus Disease in Nonhuman Primates with ZMapp." Nature (2014): n. pag. Web. 24 Apr. 2016.
- ↑ "Signs and Symptoms." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 02 Nov. 2014. Web. 24 Apr. 2016.
- ↑ Sullivan, N., Z.-Y. Yang, and G. J. Nabel. "Ebola Virus Pathogenesis: Implications for Vaccines and Therapies." Journal of Virology 77.18 (2003): 9733-737. Web. 24 Apr. 2016.
- ↑ "Treatment." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 22 July 2015. Web. 24 Apr. 2016.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
